Deck 2: The Numerical Side of Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/201
Play
Full screen (f)
Deck 2: The Numerical Side of Chemistry
1
Round off 907,506 to four significant figures.
A) 9.075 × 10-5
B) 9.075 × 105
C) 9,075
D) 90,751
E) 908,000
A) 9.075 × 10-5
B) 9.075 × 105
C) 9,075
D) 90,751
E) 908,000
9.075 × 105
2
Express the number 1.06 × 10-4 in standard notation.
A) 10,600
B) 106
C) 0.0106
D) 0.000106
A) 10,600
B) 106
C) 0.0106
D) 0.000106
0.000106
3
Convert 0.000001546 mi to scientific notation.
A) 1.546 × 106 mi
B) 1.546 × 105 mi
C) 1.546 × 10-5 mi
D) 1.546 × 10-6 mi
A) 1.546 × 106 mi
B) 1.546 × 105 mi
C) 1.546 × 10-5 mi
D) 1.546 × 10-6 mi
1.546 × 10-6 mi
4
The correct scientific notation for 147.8 × 10-3 is ________.
A) 1.478
B) 1478 × 10-4
C) 1.478 × 10-1
D) 1.478 × 10-5
A) 1.478
B) 1478 × 10-4
C) 1.478 × 10-1
D) 1.478 × 10-5

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
5
For the measured value 6.73 pounds, what is the degree of uncertainty?
A) +/- 10 pounds
B) +/- 1 pound
C) +/- 0.1 pound
D) +/- 0.01 pound
A) +/- 10 pounds
B) +/- 1 pound
C) +/- 0.1 pound
D) +/- 0.01 pound

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following represents an estimated measurement?
A) A calendar week has 7 days.
B) The U.S. national debt is 6 trillion dollars.
C) There are 50 states in the United States of America.
D) My bank account contains 19 dollars and 26 cents.
A) A calendar week has 7 days.
B) The U.S. national debt is 6 trillion dollars.
C) There are 50 states in the United States of America.
D) My bank account contains 19 dollars and 26 cents.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
7
For the final sum of 544.36 + 455.64, the number of significant figures is ________.
A) 6
B) 5
C) 4
D) 2
E) 1
A) 6
B) 5
C) 4
D) 2
E) 1

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
8
The United States has the unit of currency dollars (unit symbol: $). Which of the following is a proper expression of the value seventy seven hundred dollars +/- 1 dollar?
A) $ 7.7 × 10+4
B) $ 7.7 × 10+3
C) $ 7700
D) $ 7.700 × 10+3
A) $ 7.7 × 10+4
B) $ 7.7 × 10+3
C) $ 7700
D) $ 7.700 × 10+3

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
9
How many significant figures are in 43.8001?
A) 2
B) 3
C) 4
D) 6
A) 2
B) 3
C) 4
D) 6

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
10
The number 0.0040600 has ________ trailing zeros.
A) 0
B) 1
C) 2
D) 6
A) 0
B) 1
C) 2
D) 6

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
11
Which one of the following numbers does not have four significant figures?
A) 1.007 g
B) 0.0007 g
C) 1.070 g
D) 1700. g
E) 1.700 g
A) 1.007 g
B) 0.0007 g
C) 1.070 g
D) 1700. g
E) 1.700 g

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
12
Identify the piece of glassware that provides the least precise volume measurement of a liquid.
A) beaker
B) graduated cylinder
C) buret
D) pipette
A) beaker
B) graduated cylinder
C) buret
D) pipette

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
13
The correct answer for the operation (5.61 + 7.891 ) / 9.1 is ________.
A) 4.86
B) 4.8646714
C) 1.5
D) 5.0
A) 4.86
B) 4.8646714
C) 1.5
D) 5.0

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
14
How many significant digits are present in the number 0.02560?
A) 3
B) 4
C) 5
D) 6
A) 3
B) 4
C) 5
D) 6

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
15
By how many places must the decimal point of the number 0.0000814 be moved in order to express this same number in a standard scientific notation?
A) 4
B) 5
C) 6
D) 7
A) 4
B) 5
C) 6
D) 7

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
16
The number 0.00003007 expressed in scientific notation is ________.
A) 3.007 × 10-5
B) 3.007 × 105
C) 3.007
D) 3.007 × 104
E) 3.7 × 10-5
A) 3.007 × 10-5
B) 3.007 × 105
C) 3.007
D) 3.007 × 104
E) 3.7 × 10-5

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
17
How many significant figures are present in 0.00123, 7000, and 1.519 × 10-2?
A) 6, 4, 4
B) 4, 1, 1
C) 7, 1, 4
D) 3, 1, 4
A) 6, 4, 4
B) 4, 1, 1
C) 7, 1, 4
D) 3, 1, 4

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
18
The number 0.0247 expressed in scientific notation is ________.
A) 2.47 × 10-4
B) 2.47 × 10-2
C) 2.47 × 10-1
D) 24.7
A) 2.47 × 10-4
B) 2.47 × 10-2
C) 2.47 × 10-1
D) 24.7

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
19
Identify the exact number.
A) a number of coins
B) the diameter of a coin
C) the radius of a coin
D) the mass of a coin
A) a number of coins
B) the diameter of a coin
C) the radius of a coin
D) the mass of a coin

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
20
How many significant digits are present n the number 6046.2?
A) 4
B) 3
C) 5
D) 1
A) 4
B) 3
C) 5
D) 1

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
21
What is the prefix for the multiplier 1 × 106?
A) micro-
B) nano-
C) mega-
D) kilo-
A) micro-
B) nano-
C) mega-
D) kilo-

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
22
Using the rules of significant figures, calculate the following: 14.8903 - 2.14 =
A) 12.750
B) 12.75
C) 13
D) 12.7503
E) 12
A) 12.750
B) 12.75
C) 13
D) 12.7503
E) 12

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
23
The correct answer in the operation below is ________. (30.004 cm × 14.01 g) / 6.08 cm2
A) 69.1375 g/cm
B) 69.14 g/cm
C) 69.1 g/cm
D) 69.138 g/cm
A) 69.1375 g/cm
B) 69.14 g/cm
C) 69.1 g/cm
D) 69.138 g/cm

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
24
Which is a derived SI unit?
A) J
B) m
C) K
D) s
A) J
B) m
C) K
D) s

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
25
The sum for the operation 1.2315 + 0.116 + 15.10 is ________.
A) 16
B) 16.4
C) 16.45
D) 16.4475
A) 16
B) 16.4
C) 16.45
D) 16.4475

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following operations will not give an answer of 73.3?
A) 63.75 × 1.15
B) 90.0 - 16.67
C) 60.15 + 13.26
D) 12.161 × 6.03
A) 63.75 × 1.15
B) 90.0 - 16.67
C) 60.15 + 13.26
D) 12.161 × 6.03

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
27
What is the prefix for the multiplier 1 × 10-6?
A) micro-
B) nano-
C) mega-
D) kilo-
A) micro-
B) nano-
C) mega-
D) kilo-

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
28
10 millimeters are equal to ________ nanometers.
A) 107
B) 10-7
C) 103
D) 10-3
A) 107
B) 10-7
C) 103
D) 10-3

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
29
Which is the SI unit for temperature?
A) Fahrenheit
B) Celsius
C) Kelvin
D) either A or C
A) Fahrenheit
B) Celsius
C) Kelvin
D) either A or C

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
30
Which is the SI unit for time?
A) year
B) day
C) minute
D) second
A) year
B) day
C) minute
D) second

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
31
The prefix used to indicate 10-12 is ________.
A) micro-
B) nano-
C) pico-
D) giga-
A) micro-
B) nano-
C) pico-
D) giga-

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is not an SI unit that is used in chemistry?
A) s
B) K
C) kg
D) quart
A) s
B) K
C) kg
D) quart

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
33
How many kilograms are in 452 mg?
A) 4.52 × 10-7 kg
B) 4.52 × 10-4 kg
C) 0.452 kg
D) 4.52 × 108 kg
A) 4.52 × 10-7 kg
B) 4.52 × 10-4 kg
C) 0.452 kg
D) 4.52 × 108 kg

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following numbers expresses the value 2600. calories with both the correct units and proper scientific notation?
A) 2.6 × 104 kcal
B) 2.600 × 103 cal
C) 2.60 × 103 mcal
D) 26 × 102 dcal
A) 2.6 × 104 kcal
B) 2.600 × 103 cal
C) 2.60 × 103 mcal
D) 26 × 102 dcal

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
35
Which is the SI unit for length?
A) meter
B) inch
C) kilometer
D) mile
A) meter
B) inch
C) kilometer
D) mile

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
36
Which is not a derived SI unit?
A) Pa
B) J
C) C
D) s
A) Pa
B) J
C) C
D) s

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
37
Which is the SI unit for mass?
A) kilogram
B) pound
C) gram
D) ounce
A) kilogram
B) pound
C) gram
D) ounce

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following operations will give an answer of 50.1?
A) 29.75 + 20.3
B) 58.23 - 8.1
C) 26.255 × 1.91
D) all of the above
A) 29.75 + 20.3
B) 58.23 - 8.1
C) 26.255 × 1.91
D) all of the above

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
39
465.000 g is equivalent to ________.
A) 0.0465 kg
B) 465,000 mg
C) 4.65 kg
D) 465,000 ng
A) 0.0465 kg
B) 465,000 mg
C) 4.65 kg
D) 465,000 ng

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is defined as the quantity of matter in a sample that is independent of where it is measured?
A) weight
B) mass
C) volume
D) both A and B
A) weight
B) mass
C) volume
D) both A and B

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
41
In order to convert 121 dm to mm one needs to multiply by ________.
A) 107
B) 102
C) 10-2
D) 10-5
A) 107
B) 102
C) 10-2
D) 10-5

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is the hottest temperature?
A) 90 °C
B) 104 °F
C) 353 K
D) All of the above are the same.
A) 90 °C
B) 104 °F
C) 353 K
D) All of the above are the same.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
43
A popular science demonstration is to take several liquids that will not mix together and "stack" these liquids in a tall glass cylinder. Suppose the following three liquids were placed in the same tall, narrow glass cylinder: 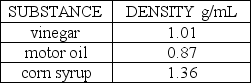 The substance that should be placed first in the cylinder so that it is on the bottom is ________.
The substance that should be placed first in the cylinder so that it is on the bottom is ________.
A) vinegar
B) motor oil
C) corn syrup
D) cannot be determined
 The substance that should be placed first in the cylinder so that it is on the bottom is ________.
The substance that should be placed first in the cylinder so that it is on the bottom is ________.A) vinegar
B) motor oil
C) corn syrup
D) cannot be determined

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
44
If the density of aluminum is 2.70 g/ cm3, the mass of 10.0 cm3 of this material is ________.
A) 27.0 g
B) 2.70 g
C) 3.70 g
D) 0.270 g
A) 27.0 g
B) 2.70 g
C) 3.70 g
D) 0.270 g

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following does not measure temperature?
A) °C
B) °F
C) K
D) J
A) °C
B) °F
C) K
D) J

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is coldest temperature?
A) -40 °C
B) -40 °F
C) 210 K
D) All of the above are the same.
A) -40 °C
B) -40 °F
C) 210 K
D) All of the above are the same.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following temperatures are not possible?
A) 2000 °C
B) -425 °F
C) -425 °C
D) 5 K
A) 2000 °C
B) -425 °F
C) -425 °C
D) 5 K

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
48
What is the density of a piece of wood that weighs 62 grams and displaces 0.525 L of water?
A) 120 g/mL
B) 0.12 g/mL
C) 0.0080 g/mL
D) 8.5 g/mL
A) 120 g/mL
B) 0.12 g/mL
C) 0.0080 g/mL
D) 8.5 g/mL

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
49
An 80.0 g sample of metal A displaces 20.0 mL of water, while a 56.0 g sample of metal B displaces 14.0 mL of water. The conclusion one may draw from this information is that ________.
A) A and B are the same metal
B) A and B are not the same metal
C) A and B may be the same metal, but more studies are needed to decide if they are the same
D) The above results tell us nothing about the nature of A and B.
A) A and B are the same metal
B) A and B are not the same metal
C) A and B may be the same metal, but more studies are needed to decide if they are the same
D) The above results tell us nothing about the nature of A and B.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
50
20.5 g of a liquid has a volume of 15 mL. What is the density?
A) 1 g/mL
B) 1.3 g/mL
C) 1.4 g/mL
D) 1.36 g/mL
A) 1 g/mL
B) 1.3 g/mL
C) 1.4 g/mL
D) 1.36 g/mL

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
51
164.0 g of a substance occupy a volume of 120.0 mL. In relation to water, which statement is true?
A) The substance will float in water.
B) The substance will sink in water.
C) The substance will react with water.
D) The substance is a metal.
A) The substance will float in water.
B) The substance will sink in water.
C) The substance will react with water.
D) The substance is a metal.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
52
Which is the lowest temperature?
A) 40 °C
B) 104 °F
C) 313 K
D) All of them are about the same.
A) 40 °C
B) 104 °F
C) 313 K
D) All of them are about the same.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
53
68 °F is equal to ________.
A) 37 °C
B) 32 °C
C) 25 °C
D) 20. °C
A) 37 °C
B) 32 °C
C) 25 °C
D) 20. °C

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
54
Ethanol has a density of 0.789 g/mL. This means that a certain volume of ethanol will ________.
A) weigh more than an equal volume of water
B) weigh less than an equal volume of water
C) weigh exactly the same as an equal volume of water
D) always weigh 0.8 g
A) weigh more than an equal volume of water
B) weigh less than an equal volume of water
C) weigh exactly the same as an equal volume of water
D) always weigh 0.8 g

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
55
The units of density for a liquid are usually expressed as ________.
A) g/mL
B) g/L
C) mg/mL
D) mg/L
A) g/mL
B) g/L
C) mg/mL
D) mg/L

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
56
40. °C is equal to ________ K.
A) 313
B) 293
C) 263
D) 233
A) 313
B) 293
C) 263
D) 233

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
57
The density of copper is about 8.96 g/mL. This means that a certain mass of copper will ________.
A) occupy the same volume as the same mass of water
B) occupy a volume 8.96 times larger than the volume occupied by the same mass of water
C) occupy a volume that is 8.96 times smaller than the volume occupied by the same mass of water
D) always occupy a volume of 8.96 mL
A) occupy the same volume as the same mass of water
B) occupy a volume 8.96 times larger than the volume occupied by the same mass of water
C) occupy a volume that is 8.96 times smaller than the volume occupied by the same mass of water
D) always occupy a volume of 8.96 mL

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
58
A liquid boils at 68 °F at one atmosphere pressure. This is equal to ________ K.
A) 341
B) 205
C) 253
D) 293
A) 341
B) 205
C) 253
D) 293

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
59
The density of a 4.0 cm3 sample of a metal is 11.3 g/cm3. What is the density of 8.0 cm3 sample of the same metal?
A) 11.3 g/ mL
B) 22.6 g/ mL
C) 5.65 g/ mL
D) 2.0 g/ mL
A) 11.3 g/ mL
B) 22.6 g/ mL
C) 5.65 g/ mL
D) 2.0 g/ mL

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
60
The correct order of decreasing temperatures in the set below is ________.
A) 18 °C > 80 °F > 280 K
B) 280 K > 18 °C > 80 °F
C) 80 °F > 18 °C > 280 K
D) 80 °F > 280 K > 18 °C
A) 18 °C > 80 °F > 280 K
B) 280 K > 18 °C > 80 °F
C) 80 °F > 18 °C > 280 K
D) 80 °F > 280 K > 18 °C

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
61
If the speed limit is 60. mi/hr, its equivalent in km/hr is ________.
A) 38
B) 97
C) 27
D) 123
A) 38
B) 97
C) 27
D) 123

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
62
20.9 kJ is equal to ________ kcal.
A) 5,000
B) 5.00
C) 873.6
D) 20
A) 5,000
B) 5.00
C) 873.6
D) 20

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
63
How many cm2 are there in 5.20 iN₂?
A) 33.5
B) 13.2
C) 2.05
D) 0.806
A) 33.5
B) 13.2
C) 2.05
D) 0.806

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
64
Convert 250.0 kcal to Joules.
A) 250.0 J
B) 5.975 × 104 J
C) 1.046 × 106 J
D) 1.046 × 103 J
A) 250.0 J
B) 5.975 × 104 J
C) 1.046 × 106 J
D) 1.046 × 103 J

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
65
Which statement is true?
A) 1 mm = 0.0394 in.
B) 1 cm3 = 1 mL
C) 1 g = 0.0022 lb
D) All of the above are true.
A) 1 mm = 0.0394 in.
B) 1 cm3 = 1 mL
C) 1 g = 0.0022 lb
D) All of the above are true.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
66
A person is 6 ft 10 in tall. This is equivalent to ________ m.
A) 2.08 m
B) 2.69 m
C) 3.22 m
D) 1.68 m
A) 2.08 m
B) 2.69 m
C) 3.22 m
D) 1.68 m

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following has the greatest mass?
A) 4.4 lb
B) 2.0 kg
C) 165 oz
D) 2.0 × 103 g
A) 4.4 lb
B) 2.0 kg
C) 165 oz
D) 2.0 × 103 g

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
68
How many moles of copper are present in 128 grams of copper (for copper, 1 mole = 63.546 grams)?
A) 0.496 moles
B) 8134 moles
C) 8.13 × 10-3 moles
D) 2.01 moles
A) 0.496 moles
B) 8134 moles
C) 8.13 × 10-3 moles
D) 2.01 moles

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
69
The closest number of grams that is equivalent to 0.745 lb is ________.
A) 338
B) 3.379
C) 0.338
D) 1.64 × 10-3
A) 338
B) 3.379
C) 0.338
D) 1.64 × 10-3

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
70
If the speed limit is 120 km/hr, its equivalent in mi/hr is ________.
A) 75
B) 192
C) 133
D) 210
A) 75
B) 192
C) 133
D) 210

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
71
The distance of the 220-yard race is equivalent to ________ ft.
A) 660
B) 2,640
C) 7.92 × 103
D) 73.3
A) 660
B) 2,640
C) 7.92 × 103
D) 73.3

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following is the shortest?
A) 18 inches
B) 0.58 m
C) 580 mm
D) 1.9 ft
A) 18 inches
B) 0.58 m
C) 580 mm
D) 1.9 ft

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
73
10. g of a certain metal absorb 40. cal of heat and the temperature is observed to rise from 20. °C to 60. °C. The specific heat of this metal is ________ cal/g °C.
A) 0.050
B) 0.10
C) 0.16
D) 0.40
A) 0.050
B) 0.10
C) 0.16
D) 0.40

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
74
500 cal are added to 10 g of water (specific heat of 1.000 cal/g °C) originally at 20 °C. The final temperature is ________.
A) 70 °C
B) 50 °C
C) 25 °C
D) 22.5 °C
A) 70 °C
B) 50 °C
C) 25 °C
D) 22.5 °C

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
75
The world record for the 100-meter dash is 9.8 s. If the world record holding runner could maintain the same pace when running five kilometers, he would cover the distance in ________.
A) 9.8 min
B) 8.2 min
C) 1.6 min
D) 5.1 min
A) 9.8 min
B) 8.2 min
C) 1.6 min
D) 5.1 min

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
76
One thousand yards are equivalent to ________.
A) 9.14 × 10-2 mm
B) 9.14 × 102 m
C) 12,000 in
D) 1,094 m
A) 9.14 × 10-2 mm
B) 9.14 × 102 m
C) 12,000 in
D) 1,094 m

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
77
5 gallons is equal to ________ quarts.
A) 5
B) 10
C) 20
D) 40
A) 5
B) 10
C) 20
D) 40

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
78
The dietary energy requirement for an adult is 2000 kcal per day. This is equivalent to ________.
A) 478 J
B) 8.37 J
C) 47.8 kJ
D) 8.37 × 103 kJ
A) 478 J
B) 8.37 J
C) 47.8 kJ
D) 8.37 × 103 kJ

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
79
The density of aluminum is 2.7 g/mL. This is equivalent to ________ kg/m3.
A) 2.7 × 104
B) 2.7
C) 0.27
D) 2.7 × 103
A) 2.7 × 104
B) 2.7
C) 0.27
D) 2.7 × 103

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
80
100. cal of heat are added to 10 g of ethanol (0.581 cal/g °C) originally at 15 °C. The final temperature is ________.
A) 17 °C
B) 27 °C
C) 32 °C
D) 38 °C
A) 17 °C
B) 27 °C
C) 32 °C
D) 38 °C

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck


