Deck 9: Addition Reactions of Alkenes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/148
Play
Full screen (f)
Deck 9: Addition Reactions of Alkenes
1
The expected major product of the following reaction is: 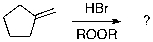
A)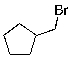
B)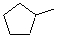
C)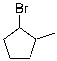
D)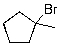
E)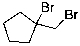
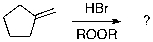
A)

B)

C)

D)

E)


2
Which of the alkenes below would be expected to produce at least one chirality center upon hydrohalogenation in the presence of peroxide?
A)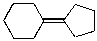
B)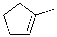
C)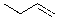
D)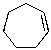
E)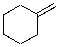
A)

B)

C)
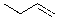
D)

E)


3
Addition reactions of alkenes are characterized by:
A) formation of a π bond
B) addition of two groups across a double bond
C) breaking of a π bond
D) A and B
E) B and C
A) formation of a π bond
B) addition of two groups across a double bond
C) breaking of a π bond
D) A and B
E) B and C
B and C
4
What is the major product for the following reaction? 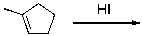
A)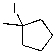
B)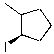
C)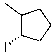
D)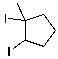
E)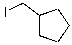
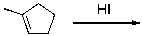
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
5
What is the expected major product for the following reaction? 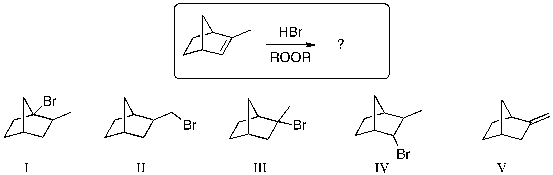
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the molecules below arises from anti-Markovnikov hydrohalogenation with HBr of the alkene shown? 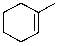
A)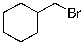
B)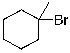
C)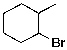
D)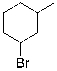
E)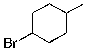

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the molecules below would be the expected product for hydrohalogenation of the following alkene with HBr: 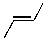
A)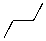
B)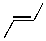
C)
D)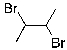
E)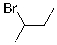
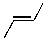
A)

B)
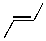
C)

D)

E)
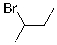

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
8
The expected major product of the following reaction is: 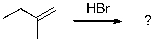
A)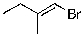
B)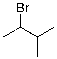
C)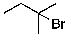
D)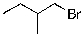
E)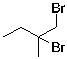
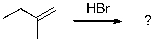
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
9
In a reaction where addition and elimination reactions are in equilibrium, which of the following statements is most correct?
A) Addition and elimination reactions are favored at low temperatures.
B) Addition and elimination reactions are favored at high temperatures.
C) Only addition reactions are favored at low temperatures.
D) Only elimination reactions are favored at low temperatures.
E) Addition and elimination reactions are disfavored at low temperatures.
A) Addition and elimination reactions are favored at low temperatures.
B) Addition and elimination reactions are favored at high temperatures.
C) Only addition reactions are favored at low temperatures.
D) Only elimination reactions are favored at low temperatures.
E) Addition and elimination reactions are disfavored at low temperatures.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
10
The decrease in entropy (the S value is negative) observed for alkene addition reactions results from:
A) the breaking of a π and bond.
B) the formation of two bonds.
C) the reaction being exothermic.
D) two molecules reacting to form a single molecule.
E) the temperature dependence of the S term.
A) the breaking of a π and bond.
B) the formation of two bonds.
C) the reaction being exothermic.
D) two molecules reacting to form a single molecule.
E) the temperature dependence of the S term.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the alkenes shown below would produce a chirality center upon Markovnikov hydrohalogenation?
A)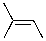
B)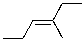
C)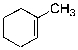
D)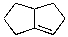
E)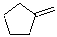
A)
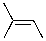
B)
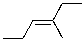
C)

D)

E)


Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
12
In an addition reaction to an alkene, the π bond plays the role of:
A) nucleophile
B) electrophile
C) leaving group
D) A and B
E) B and C
A) nucleophile
B) electrophile
C) leaving group
D) A and B
E) B and C

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
13
In the addition reaction of HI to 2-methyl-2-butene, the Markovnikov addition mechanism involves:
A) attack of 2-methyl-2-butene initiated by an iodide ion.
B) attack of 2-methyl-2-butene initiated by an iodine atom.
C) isomerization of 2-iodo-2-methylbutene.
D) formation of a carbocation at carbon two (C-2).
E) formation of carbocation at carbon three (C-3).
A) attack of 2-methyl-2-butene initiated by an iodide ion.
B) attack of 2-methyl-2-butene initiated by an iodine atom.
C) isomerization of 2-iodo-2-methylbutene.
D) formation of a carbocation at carbon two (C-2).
E) formation of carbocation at carbon three (C-3).

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the structures shown depicts the most stable carbocation intermediate formed in the hydrohalogenation reaction shown? 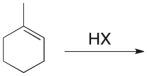
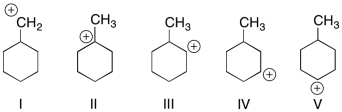
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
15
What compound is the expected product upon Markovnikov hydrohalogenation with HBr of the alkene shown below? 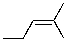
A)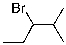
B)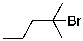
C)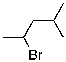
D)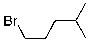
E)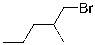
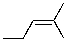
A)

B)
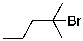
C)

D)

E)


Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
16
The regioselectivity and stereospecificity in hydrohalogenation of an alkene is best described as:
A) Markovnikov orientation with syn-addition
B) Markovnikov orientation with anti-addition
C) anti-Markovnikov orientation with syn-addition
D) anti-Markovnikov orientation with anti-addition
E) Markovnikov orientation with both syn- and anti-addition
A) Markovnikov orientation with syn-addition
B) Markovnikov orientation with anti-addition
C) anti-Markovnikov orientation with syn-addition
D) anti-Markovnikov orientation with anti-addition
E) Markovnikov orientation with both syn- and anti-addition

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
17
For an addition reaction, why does free energy, G, become more positive with increasing temperature?
A) The positive entropy dominates at high temperature.
B) The negative entropy dominates at high temperature.
C) The positive enthalpy dominates at high temperature.
D) The negative enthalpy dominates at high temperature.
E) The enthalpy and entropy cancel at high temperature.
A) The positive entropy dominates at high temperature.
B) The negative entropy dominates at high temperature.
C) The positive enthalpy dominates at high temperature.
D) The negative enthalpy dominates at high temperature.
E) The enthalpy and entropy cancel at high temperature.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
18
For this question, the "entropy term" refers to "-TΔS". Addition reactions are generally favorable at low temperatures because:
A) the positive enthalpy term is larger than the negative entropy term.
B) the negative enthalpy term is larger than the positive entropy term.
C) the positive enthalpy term is smaller than the negative entropy term.
D) the negative enthalpy term is smaller than the positive entropy term.
E) the enthalpy and entropy terms are equal.
A) the positive enthalpy term is larger than the negative entropy term.
B) the negative enthalpy term is larger than the positive entropy term.
C) the positive enthalpy term is smaller than the negative entropy term.
D) the negative enthalpy term is smaller than the positive entropy term.
E) the enthalpy and entropy terms are equal.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
19
Which statement best describes the temperature dependence of an addition reaction?
A) Addition reactions are thermodynamically favored at all temperatures.
B) Addition reactions are thermodynamically disfavored at all temperatures.
C) Addition reactions are thermodynamically favored at low temperatures.
D) Addition reactions are thermodynamically favored at high temperatures.
E) Addition reactions are thermodynamically impossible.
A) Addition reactions are thermodynamically favored at all temperatures.
B) Addition reactions are thermodynamically disfavored at all temperatures.
C) Addition reactions are thermodynamically favored at low temperatures.
D) Addition reactions are thermodynamically favored at high temperatures.
E) Addition reactions are thermodynamically impossible.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
20
The expected Markovnikov addition product of HI to 2-methyl-2-butene is:
A) 2-iodopentane
B) 2-iodo-2-methylbutane
C) 1-iodo-2-methylbutane
D) 1-iodo-3-methylbutane
E) 2-iodo-3-methylbutane
A) 2-iodopentane
B) 2-iodo-2-methylbutane
C) 1-iodo-2-methylbutane
D) 1-iodo-3-methylbutane
E) 2-iodo-3-methylbutane

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
21
Treatment of 1-methylcyclohexene with H3O+ would be expected to yield as the major product which of the molecules below? 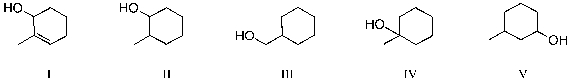
A) I and III
B) II
C) III and V
D) IV
E) I, III and V

A) I and III
B) II
C) III and V
D) IV
E) I, III and V

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
22
The expected major product of the following reaction is: 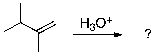
A)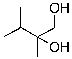
B)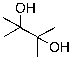
C)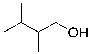
D)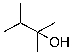
E)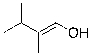
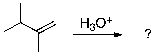
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
23
The expected major product(s) of HBr addition to the alkene shown would be: 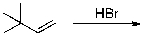
A)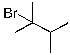
B)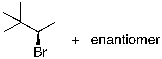
C)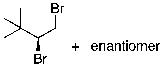
D)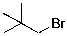
E)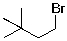
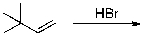
A)

B)
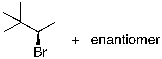
C)
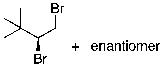
D)

E)


Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
24
What is the expected major product for the following reaction? 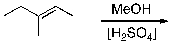
A)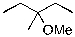
B)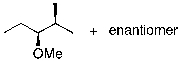
C)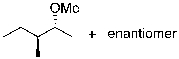
D)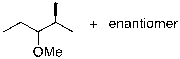
E)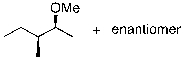
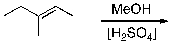
A)
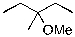
B)
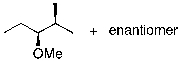
C)
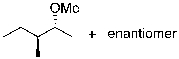
D)
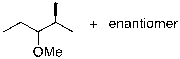
E)
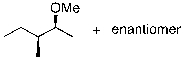

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
25
The expected major product of the following reaction is: 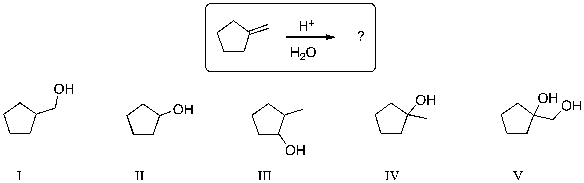
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
26
Select the expected major product(s) of the following reaction: 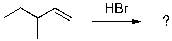
A)
B)
C)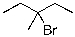
D) A and B
E) A and C
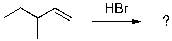
A)

B)

C)

D) A and B
E) A and C

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
27
What would be the optimal conditions to effect the following transformation? 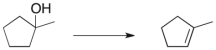
A) Dilute H2SO4
B) Concentrated H2SO4
C) Dilute HBr
D) Concentrated HBr

A) Dilute H2SO4
B) Concentrated H2SO4
C) Dilute HBr
D) Concentrated HBr

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following carbocations is (are) likely to undergo rearrangement through a hydride shift? 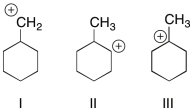
A) I
B) II
C) III
D) I and II
E) II and III

A) I
B) II
C) III
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following carbocations is (are) likely to undergo rearrangement through a methyl shift? 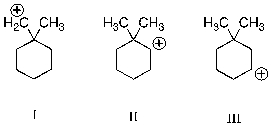
A) I
B) II
C) III
D) I and II
E) II and III

A) I
B) II
C) III
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following compounds would react most rapidly with a dilute aqueous solution of H2SO4? 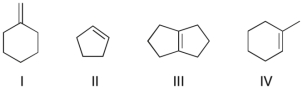
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
31
The reaction shown below would be expected to produce as major products which of the following compounds? 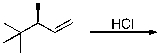
A)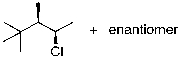
B)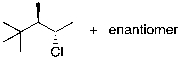
C)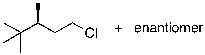
D)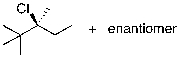
E)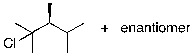
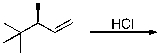
A)
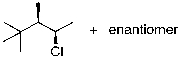
B)
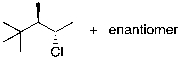
C)
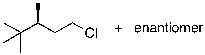
D)
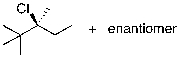
E)
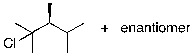

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
32
What is the IUPAC name of the expected major product formed upon reaction of HCl with 3-methyl-1-butene?
A) 1-chloro-2-methylbutane
B) 1-chloro-3-methylbutane
C) 2-chloro-2-methylbutane
D) 2-chloro-3-methylbutane
E) 1-chloropentane
A) 1-chloro-2-methylbutane
B) 1-chloro-3-methylbutane
C) 2-chloro-2-methylbutane
D) 2-chloro-3-methylbutane
E) 1-chloropentane

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
33
Identify the expected product(s) for the reaction shown below: 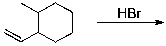
A)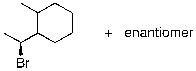
B)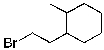
C)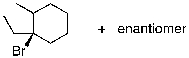
D)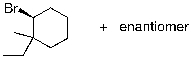
E)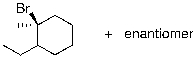
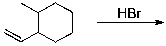
A)
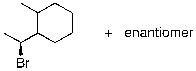
B)

C)
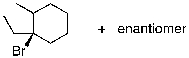
D)
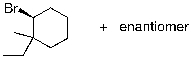
E)
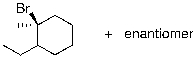

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the given reaction schemes would produce the molecule shown below? 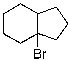
A)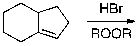
B)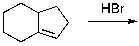
C)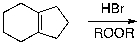
D) Both A and B
E) Both B and C

A)
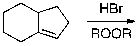
B)
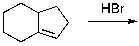
C)
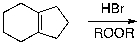
D) Both A and B
E) Both B and C

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
35
The regioselectivity and stereospecificity in the acid-catalyzed hydration of an alkene is best described as:
A) Markovnikov orientation with syn-addition
B) Markovnikov orientation with anti-addition
C) anti-Markovnikov orientation with syn-addition
D) anti-Markovnikov orientation with anti-addition
E) Markovnikov orientation with both syn- and anti-addition
A) Markovnikov orientation with syn-addition
B) Markovnikov orientation with anti-addition
C) anti-Markovnikov orientation with syn-addition
D) anti-Markovnikov orientation with anti-addition
E) Markovnikov orientation with both syn- and anti-addition

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
36
What would be the optimal conditions to effect the following transformation? 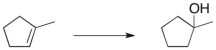
A) Dilute aqueous H2SO4
B) Concentrated H2SO4
C) Dilute aqueous HBr
D) Concentrated HBr

A) Dilute aqueous H2SO4
B) Concentrated H2SO4
C) Dilute aqueous HBr
D) Concentrated HBr

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the molecules below are enantiomers formed as products upon reaction of HBr with 4-methylpent-1-ene? 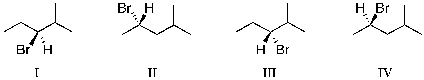
A) I and II
B) II and III
C) III and IV
D) I and III
E) II and IV

A) I and II
B) II and III
C) III and IV
D) I and III
E) II and IV

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
38
The three compounds below can form a carbocation under aqueous acidic conditions. Which ones will form the same carbocation? 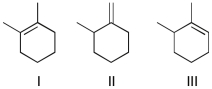
A) I and II
B) I and III
C) II and III
D) All three will form the same carbocation

A) I and II
B) I and III
C) II and III
D) All three will form the same carbocation

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
39
The expected major product(s) of HCl addition to the alkene shown would be: 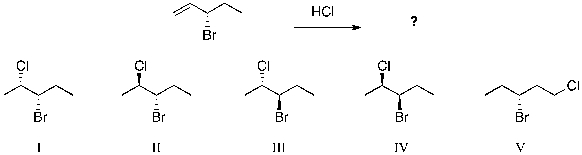
A) II
B) II and III
C) I and IV
D) V
E) All of the above are equally likely to form

A) II
B) II and III
C) I and IV
D) V
E) All of the above are equally likely to form

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
40
The expected major product of the following reaction is: 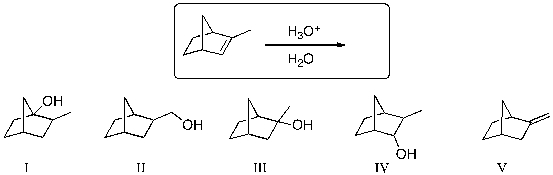
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
41
What is the expected product for the hydrogenation of an alkene?
A) dihaloalkane
B) alkane
C) haloalkane
D) alcohol
E) ether
A) dihaloalkane
B) alkane
C) haloalkane
D) alcohol
E) ether

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
42
The regioselectivity and stereospecificity in the oxymercuration of an alkene is best described as:
A) Markovnikov orientation with syn-addition
B) Markovnikov orientation with anti-addition
C) anti-Markovnikov orientation with syn-addition
D) anti-Markovnikov orientation with anti-addition
E) Markovnikov orientation with both syn- and anti-addition
A) Markovnikov orientation with syn-addition
B) Markovnikov orientation with anti-addition
C) anti-Markovnikov orientation with syn-addition
D) anti-Markovnikov orientation with anti-addition
E) Markovnikov orientation with both syn- and anti-addition

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
43
What is the expected major product for the following reaction sequence? 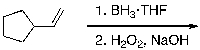
A)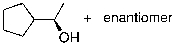
B)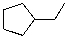
C)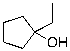
D)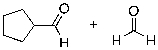
E)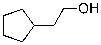
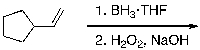
A)
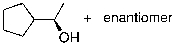
B)

C)

D)
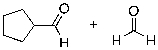
E)


Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
44
What is the expected major product for the following reaction? 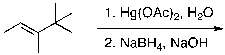
A)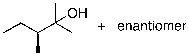
B)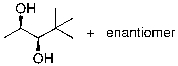
C)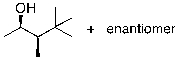
D)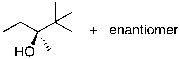
E)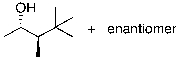
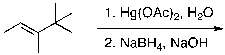
A)
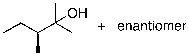
B)
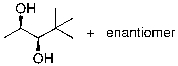
C)
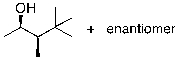
D)
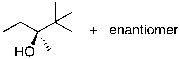
E)
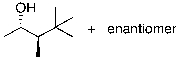

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is the major product of the reaction shown? 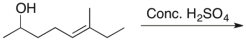
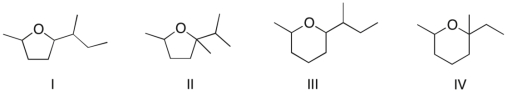
A) I
B) II
C) III
D) IV


A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
46
When an alkene is subjected to treatment with Hg(OAc)2 in alcohol, followed by reaction with NaBH4, what functional group is formed?
A) ether
B) epoxide
C) alkane
D) syn diol
E) alkyne
A) ether
B) epoxide
C) alkane
D) syn diol
E) alkyne

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
47
What reagents are needed to accomplish the following transformation? 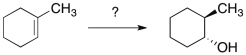
A) H2O/H+
B) H2O/Peroxide
C) OH-
D) BH3.THF
E) 1. BH3.THF, 2. HO-, H2O2, H2O

A) H2O/H+
B) H2O/Peroxide
C) OH-
D) BH3.THF
E) 1. BH3.THF, 2. HO-, H2O2, H2O

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
48
What is (are) the expected major product(s) for the following reaction sequence? 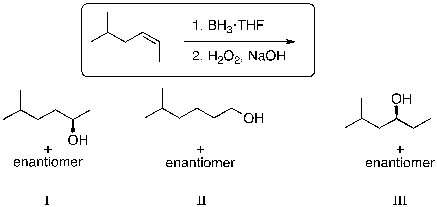
A) I
B) II
C) III
D) I and II
E) I and III

A) I
B) II
C) III
D) I and II
E) I and III

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
49
What is the expected major product for the following reaction? 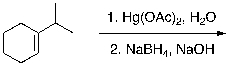
A)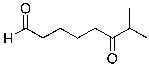
B)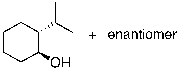
C)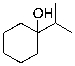
D)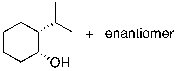
E)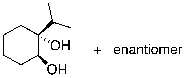
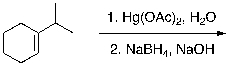
A)

B)
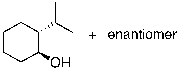
C)

D)
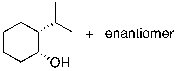
E)

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
50
What is the expected major product for the following reaction sequence? 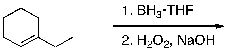
A)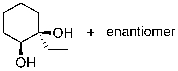
B)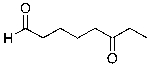
C)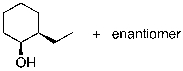
D)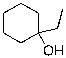
E)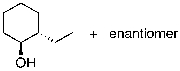
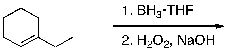
A)
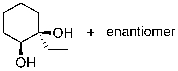
B)

C)
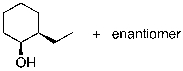
D)

E)

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following correctly describes the product(s) of the reaction below? 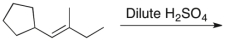
A) Only one stereoisomer
B) An equal mixture of enantiomers
C) A roughly equal mixture of diasteromers
D) A roughly equal mixture of constitutional isomers
A) Only one stereoisomer
B) An equal mixture of enantiomers
C) A roughly equal mixture of diasteromers
D) A roughly equal mixture of constitutional isomers

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
52
What product is formed when trans-1,2-dimethylcyclohexane is reacted with Pd/C and H2?
A) No reaction
B) cis-1,2-dimethylcyclohexane
C) trans-3,4-dimethylhexane
D) trans-1,2-dimethylhexane
E) None of the above
A) No reaction
B) cis-1,2-dimethylcyclohexane
C) trans-3,4-dimethylhexane
D) trans-1,2-dimethylhexane
E) None of the above

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
53
What is the expected major product for the following reaction sequence? 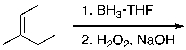
A)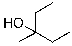
B)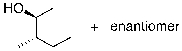
C)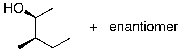
D)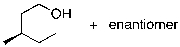
E)
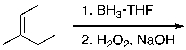
A)

B)
C)
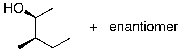
D)
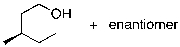
E)


Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
54
What is the expected major product for the following reaction? 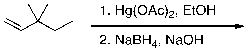
A)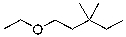
B)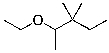
C)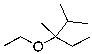
D)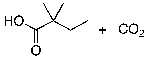
E)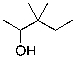
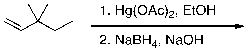
A)

B)

C)

D)
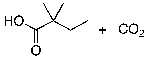
E)


Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
55
What is the expected major product for the following reaction? 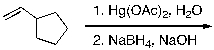
A)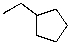
B)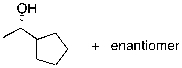
C)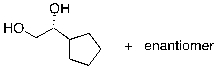
D)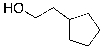
E)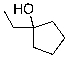
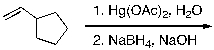
A)

B)
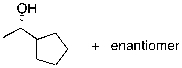
C)
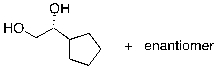
D)

E)


Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
56
What synthetic goal is achieved by subjecting an alkene to an oxymercuration-demercuration reaction sequence?
A) Markovnikov addition of H2O, promoting skeletal rearrangement
B) Markovnikov addition of H2O, preventing skeletal rearrangement
C) anti-Markovnikov addition of H2O, promoting skeletal rearrangement
D) anti-Markovnikov addition of H2O, preventing skeletal rearrangement
E) anti-Markovnikov addition of H2O, syn-hydroxylation
A) Markovnikov addition of H2O, promoting skeletal rearrangement
B) Markovnikov addition of H2O, preventing skeletal rearrangement
C) anti-Markovnikov addition of H2O, promoting skeletal rearrangement
D) anti-Markovnikov addition of H2O, preventing skeletal rearrangement
E) anti-Markovnikov addition of H2O, syn-hydroxylation

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
57
What is the name of the product formed when 5-chloro-1-methylcyclohexene is reduced with a Pt catalyst and H2?
A) 1-chloro-2-methylcyclohexane
B) 1-chloro-3-methylcyclohexane
C) 1-chloro-5-methylcyclohexane
D) 3-chloro-1-methylcyclohexane
E) None of the above
A) 1-chloro-2-methylcyclohexane
B) 1-chloro-3-methylcyclohexane
C) 1-chloro-5-methylcyclohexane
D) 3-chloro-1-methylcyclohexane
E) None of the above

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
58
The regioselectivity and stereospecificity in the hydroboration-oxidation of an alkene is best described as:
A) Markovnikov orientation with syn-addition
B) Markovnikov orientation with anti-addition
C) Anti-Markovnikov orientation with syn-addition
D) Anti-Markovnikov orientation with anti-addition
E) Markovnikov orientation with both syn- and anti-addition
A) Markovnikov orientation with syn-addition
B) Markovnikov orientation with anti-addition
C) Anti-Markovnikov orientation with syn-addition
D) Anti-Markovnikov orientation with anti-addition
E) Markovnikov orientation with both syn- and anti-addition

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
59
Provide the organic product(s) for the reaction shown below. 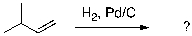
A)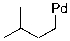
B)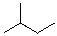
C)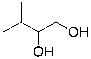
D)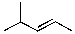
E)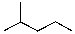
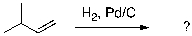
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
60
What is the expected major product for the following reaction sequence? 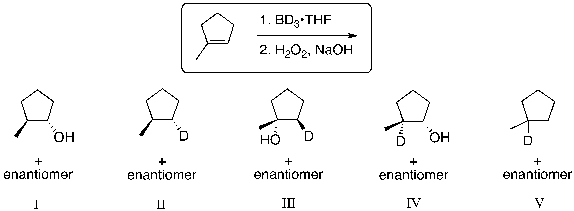
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
61
What is the expected major product upon reaction of 1-pentene with Cl2?
A) 1-chloropentane
B) 2-chloropentane
C) 1,1-dichloropentane
D) 2,2-dichloropentane
E) 1,2-dichloropentane
A) 1-chloropentane
B) 2-chloropentane
C) 1,1-dichloropentane
D) 2,2-dichloropentane
E) 1,2-dichloropentane

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
62
For the complete reduction of 1 mole of (3E,5E)-hepta-1,3,5-triene with H2 using a Pd catalyst, how many moles of H2 are consumed?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
63
Wilkinson's catalyst accomplishes which of the listed molecular transformations?
A) syn addition of H2 to an alkene
B) anti addition of H2 to an alkene
C) syn dihydroxylation of an alkene
D) anti dihydroxylation of an alkene
E) oxidative cleavage of an alkene
A) syn addition of H2 to an alkene
B) anti addition of H2 to an alkene
C) syn dihydroxylation of an alkene
D) anti dihydroxylation of an alkene
E) oxidative cleavage of an alkene

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the catalysts listed are used in the homogenous catalytic hydrogenation of alkenes? I) Ni II) Pt III) Wilkinson's catalyst
A) I
B) II
C) III
D) I and II
E) II and III
A) I
B) II
C) III
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
65
Treating 2-methyl-2-pentene with Br2 is expected to produce which of the following as the major product?
A) 2,3-dibromo-2-methylpentane
B) 2,2-dibromo-3-methylpentane
C) 3,3-dibromo-2-methylpentane
D) 2-bromo-2-methylpentane
E) 3-bromo-2-methylpentane
A) 2,3-dibromo-2-methylpentane
B) 2,2-dibromo-3-methylpentane
C) 3,3-dibromo-2-methylpentane
D) 2-bromo-2-methylpentane
E) 3-bromo-2-methylpentane

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
66
The expected first intermediate formed during a halohydrin reaction is:
A) a halonium ion.
B) the most stable carbocation with OH on the adjacent carbon.
C) the most stable carbocation with X on the adjacent carbon.
D) a cyclic oxonium ion.
E) the most stable carbanion.
A) a halonium ion.
B) the most stable carbocation with OH on the adjacent carbon.
C) the most stable carbocation with X on the adjacent carbon.
D) a cyclic oxonium ion.
E) the most stable carbanion.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
67
What is the expected major product for the following reaction? 
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the statements is most correct regarding the products expected from the halogenation reaction shown below? 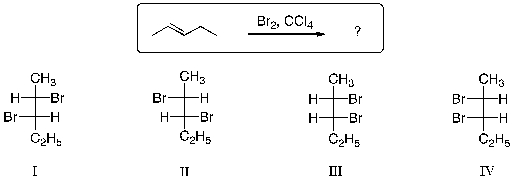
A) Equal amounts of I and II are produced.
B) Equal amounts of III and IV are produced.
C) Equal amounts of I and IV are produced.
D) Equal amounts of II and III are produced.
E) Equal amounts of I and III are produced.

A) Equal amounts of I and II are produced.
B) Equal amounts of III and IV are produced.
C) Equal amounts of I and IV are produced.
D) Equal amounts of II and III are produced.
E) Equal amounts of I and III are produced.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
69
The reaction of Br2 with 1-methylcyclohexene, in the presence of ethylamine (EtNH2), is expected to produce which of the following as the major product? 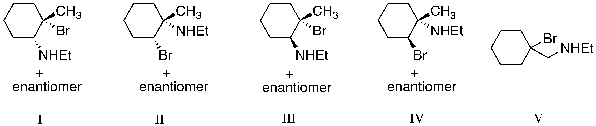
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
70
How many moles of hydrogen (H2) are consumed in the catalytic reduction of 1 mole of 1,3-dibromocyclohexa-1,4-diene?
A) 12
B) 2
C) 3
D) 4
E) None of the above
A) 12
B) 2
C) 3
D) 4
E) None of the above

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
71
What major product is expected from the following reaction? 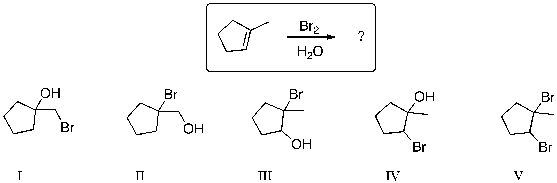
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
72
How many moles of hydrogen (H2) are required to completely reduce 1 mole of cis-2,3,3-trimethylhepta-1,5-diene?
A) 02
B) 1
C) 2
D) 3
E) 4
A) 02
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
73
In conducting a catalytic hydrogenation of an alkene, which catalyst listed is most likely soluble in the reaction medium?
A) Ni
B) Pt
C) Pd
D) Wilkinson's catalyst
E) All of the above are soluble
A) Ni
B) Pt
C) Pd
D) Wilkinson's catalyst
E) All of the above are soluble

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following sets of reagents accomplishes the transformation shown below? 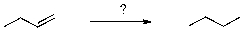
A) H2/HCl
B) H2/H2SO4
C) H2/Ni
D) H2O/Ni
E) H2O/H2SO4
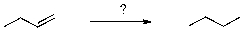
A) H2/HCl
B) H2/H2SO4
C) H2/Ni
D) H2O/Ni
E) H2O/H2SO4

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
75
Identify the major organic product generated from the reaction shown. 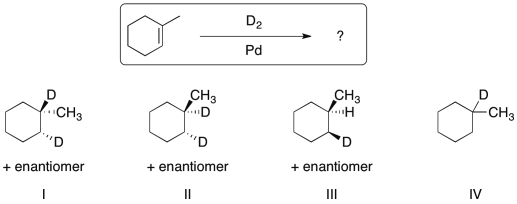
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
76
Which alkene would yield 3-methylpentane upon subjection to catalytic hydrogenation? 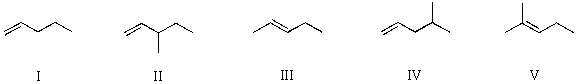
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
77
For the catalytic hydrogenation of alkenes, select from below those most likely to function as a homogenous catalyst?
A) Wilkinson's catalyst
B) Ni
C) Pd
D) Pt
E) Au
A) Wilkinson's catalyst
B) Ni
C) Pd
D) Pt
E) Au

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
78
Which reaction intermediate is formed when 4?methylcyclohexene reacts with Br2 dissolved in CCl4? 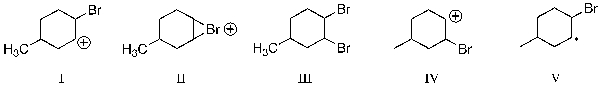
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following will yield 2-methylpentane upon catalytic hydrogenation?
A) 2-methyl-1-pentene2
B) 2-methyl-2-pentene
C) 4-methyl-2-pentene
D) 4-methyl-1-pentene
E) All of the above
A) 2-methyl-1-pentene2
B) 2-methyl-2-pentene
C) 4-methyl-2-pentene
D) 4-methyl-1-pentene
E) All of the above

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the reagents below are expected to convert cyclopentene into cyclopentane?
A) H2 and Ni
B) H2O
C) Heat
D) Zn, H3O+
E) Light
A) H2 and Ni
B) H2O
C) Heat
D) Zn, H3O+
E) Light

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck


