Deck 22: Condensations and Alpha Substitutions of Carboxyl Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/127
Play
Full screen (f)
Deck 22: Condensations and Alpha Substitutions of Carboxyl Compounds
1
For the equilibrium shown below, is the Keq greater or less than 1? 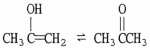
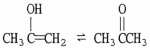
greater than 1
2
The relationship between ketones and their corresponding enols is one of ________.
A) allotropes
B) tautomers
C) enantiomers
D) diastereomers
E) cis-trans isomers
A) allotropes
B) tautomers
C) enantiomers
D) diastereomers
E) cis-trans isomers
tautomers
3
Which of the pairs shown below are tautomers?
A)
B)
C)
D)
A)

B)

C)

D)


4
Provide the structure of the more stable enol tautomer of 1-phenyl-2-octanone.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
5
Provide a detailed, stepwise mechanism for the acid-catalyzed enolization of acetaldehyde.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
6
Provide a detailed, stepwise mechanism for the base-catalyzed enolization of acetaldehyde.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following compounds will not undergo reaction with an enolate?
A) 1-pentene
B) bromine
C) propanal
D) 1-bromobutane
E) benzoyl chloride
A) 1-pentene
B) bromine
C) propanal
D) 1-bromobutane
E) benzoyl chloride

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
8
Consider the reaction shown below. Use pKa values to determine which way the equilibrium lies. 


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
9
What type of reaction is shown below? 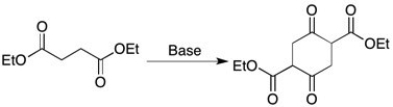
A) Aldol condensation
B) Claisen Condensation
C) Michael Addition
D) Alkylation

A) Aldol condensation
B) Claisen Condensation
C) Michael Addition
D) Alkylation

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
10
Provide the structure of lithium diisopropylamide.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
11
Draw the important resonance forms for the enolate ion/s that are formed when acetophenone reacts with base.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
12
Disregarding stereoisomers, how many different enols can the β-diketone CH3COCH2COCH2CH3 form?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
13
Provide the major organic product of the following reaction. 


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
14
Provide the structure of the enol of 2,2,4-trimethyl-3-pentanone.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following reagents will quantitatively convert an enolizable ketone to its enolate salt?
A) lithium hydroxide
B) lithium diisopropylamide
C) methyllithium
D) diethylamine
E) pyridine
A) lithium hydroxide
B) lithium diisopropylamide
C) methyllithium
D) diethylamine
E) pyridine

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
16
The reaction of LDA with acetophenone produces ________.
A) an enol
B) an enolate
C) an ylide
D) alkylation
E) halogenation
A) an enol
B) an enolate
C) an ylide
D) alkylation
E) halogenation

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
17
When 2-methylcyclohexanone is treated with catalytic base in excess D2O, how many deuterium atoms become incorporated in the organic compound?
A) 0
B) 1
C) 2
D) 3
E) 5
A) 0
B) 1
C) 2
D) 3
E) 5

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
18
Show how an enolate can add to a carbonyl.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
19
Consider the reaction shown below. Use pKa values to determine which way the equilibrium lies. 


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
20
Disregarding stereoisomers, how many different enols can phenylacetone form?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
21
Provide the major organic product of the following reaction. 


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
22
What iminium salt is produced in the reaction shown below? 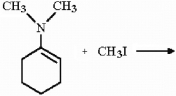


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
23
What alkyl halide would you add to perform the following alkylation reaction? 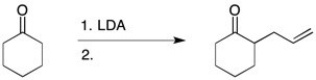


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
24
What type of product results when 3-pentanone reacts with dimethylamine?
A) enolate
B) enol
C) amide
D) imine
E) enamine
A) enolate
B) enol
C) amide
D) imine
E) enamine

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
25
(S)-2-Methylbutanal ________ upon sitting in an acidic or a basic aqueous solution.
A) racemizes
B) esterifies
C) inverts completely to the R configuration
D) hydrolyzes
E) irreversibly forms the hydrate
A) racemizes
B) esterifies
C) inverts completely to the R configuration
D) hydrolyzes
E) irreversibly forms the hydrate

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
26
Predict the major organic product of the following reaction: 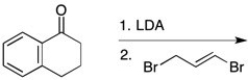


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
27
When a ketone and its enol are in equilibrium, under most conditions the concentration of the enol is ________ the concentration of the ketone.
A) slightly higher than
B) equal to
C) much higher than
D) much lower than
E) exactly half of
A) slightly higher than
B) equal to
C) much higher than
D) much lower than
E) exactly half of

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following ketones will give a positive iodoform test?
A) 4-heptanone
B) 3-hexanone
C) 2-hexanone
D) cyclohexanone
E) 2-methyl-3-pentanone
A) 4-heptanone
B) 3-hexanone
C) 2-hexanone
D) cyclohexanone
E) 2-methyl-3-pentanone

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
29
What compound is produced in the reaction of cyclopentanone with Br2 in acetic acid?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following will alkylate a lithium enolate most rapidly?
A) methyl bromide
B) isopropyl bromide
C) neopentyl bromide
D) bromobenzene
E) 2-methylbromobenzene
A) methyl bromide
B) isopropyl bromide
C) neopentyl bromide
D) bromobenzene
E) 2-methylbromobenzene

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
31
The α-halogenation of cyclohexanone ________.
A) is catalyzed by base
B) is slowed by the presence of acid
C) requires one equivalent of base
D) requires one equivalent of acid
E) is catalyzed by the sodium halide salt
A) is catalyzed by base
B) is slowed by the presence of acid
C) requires one equivalent of base
D) requires one equivalent of acid
E) is catalyzed by the sodium halide salt

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
32
What is the carbon nucleophile which attacks molecular bromine in the acid-catalyzed α-bromination of a ketone?
A) an enolate
B) a Grignard reagent
C) an acetylide
D) a carbocation
E) an enol
A) an enolate
B) a Grignard reagent
C) an acetylide
D) a carbocation
E) an enol

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
33
Provide the major organic product of the following reaction. 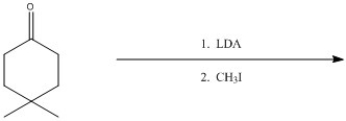


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following will react most slowly with an enamine?
A) isopropyl chloride
B) methyl bromide
C) acetyl chloride
D) benzyl chloride
E) allyl bromide
A) isopropyl chloride
B) methyl bromide
C) acetyl chloride
D) benzyl chloride
E) allyl bromide

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
35
When aldehydes are subjected to the same conditions that α-halogenate ketones (i.e., X2 and aqueous acid or base), they are ________.
A) α-halogenated as well
B) reduced to alcohols
C) converted to the acid halide
D) oxidized to the acid or carboxylate
E) esterified
A) α-halogenated as well
B) reduced to alcohols
C) converted to the acid halide
D) oxidized to the acid or carboxylate
E) esterified

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
36
What are the two key resonance structures for an enamine? Label the major and minor contributors.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
37
Provide the major organic product of the reaction shown below. 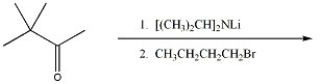


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
38
In the iodoform reaction, a methyl ketone is converted to the ________ upon treatment with excess iodine and hydroxide.
A) carboxylate
B) acyl iodide
C) primary alkyl iodide
D) aldehyde
E) primary amide
A) carboxylate
B) acyl iodide
C) primary alkyl iodide
D) aldehyde
E) primary amide

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
39
Provide the major organic product of the following reaction. 


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
40
Provide the major organic product of the reaction shown below. 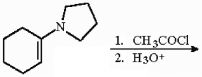


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
41
Provide the structure of the major organic product which results when PhCH2CHO is treated with NaOH.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
42
Provide the structure of the aldol product that results when 5-methylhexanal is treated with hydroxide.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
43
Show how the following compound can be synthesized using an Aldol Condensation reaction. 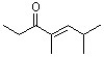


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
44
What reagents are needed to complete the following synthesis? 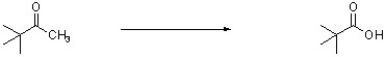
A) 1) NaOH / heat, 2) HCl (aq)
B) 1) NaOH / I2, 2) HCl (aq)
C) 1) warm conc. KMnO4 / NaOH, 2) HCl (aq)
D) 1) Ag(NH3)2OH, 2) HCl (aq)

A) 1) NaOH / heat, 2) HCl (aq)
B) 1) NaOH / I2, 2) HCl (aq)
C) 1) warm conc. KMnO4 / NaOH, 2) HCl (aq)
D) 1) Ag(NH3)2OH, 2) HCl (aq)

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is another name for the product of an aldol condensation?
A) β-hydroxyaldehyde
B) α-hydroxyaldehyde
C) acetal
D) β-ketoester
E) 1,3-dialdehyde
A) β-hydroxyaldehyde
B) α-hydroxyaldehyde
C) acetal
D) β-ketoester
E) 1,3-dialdehyde

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
46
Provide the major organic product of the following reaction. 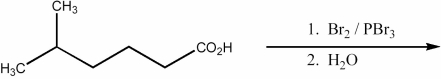


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
47
Predict the major organic product of the following reaction: 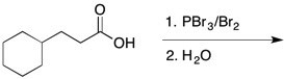


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
48
Complete the following short synthesis by providing the necessary sequence of reagents. 


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
49
Provide a mechanism for the following reaction:
butanoic acid + Br2 + PBr3 → 2-bromobutanoyl bromide
butanoic acid + Br2 + PBr3 → 2-bromobutanoyl bromide

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
50
Provide a detailed, stepwise mechanism for the formation of acetate and bromodiiodomethane from bromoacetone, hydroxide and iodine.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
51
Complete the following reaction by filling in the necessary reagents. 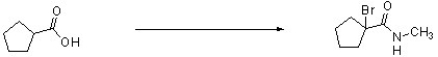


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
52
Why is the acid-catalyzed halogenation of ketones generally preferred over the base-promoted halogenation?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
53
Provide the major organic product of the following reaction. 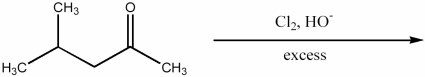


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
54
The following compound was found to inhibit HIV-1 (J. Med. Chem. 2011, 1812). Predict the structure of the hydrolysis product. 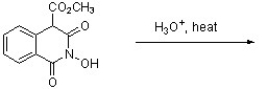
A)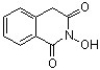
B)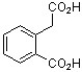
C)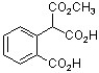
D)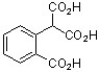

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
55
The aldol condensation is ________.
A) an irreversible reaction
B) an equilibrium reaction
C) a tautomerization
D) an isomerization
E) a type of esterification
A) an irreversible reaction
B) an equilibrium reaction
C) a tautomerization
D) an isomerization
E) a type of esterification

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
56
An enolate attacks an aldehyde and the resulting product is subsequently protonated. What type of reaction is this?
A) a Fischer esterification
B) an acid-catalyzed aldol condensation
C) a base-mediated aldol condensation
D) a Hell-Volhard-Zelinsky reaction
E) a Selman-Jones reaction
A) a Fischer esterification
B) an acid-catalyzed aldol condensation
C) a base-mediated aldol condensation
D) a Hell-Volhard-Zelinsky reaction
E) a Selman-Jones reaction

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
57
What organic compounds are produced when 2-pentanone undergoes the haloform reaction upon treatment with HO- and excess Br2?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
58
The Hell-Volhard-Zelinsky reaction involves ________.
A) the α-bromination of carboxylic acids
B) the α-bromination of ketones
C) the bromination of alcohols
D) the oxidation of aldehydes to acids
E) none of the above
A) the α-bromination of carboxylic acids
B) the α-bromination of ketones
C) the bromination of alcohols
D) the oxidation of aldehydes to acids
E) none of the above

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
59
Provide the major organic product of the following reaction. 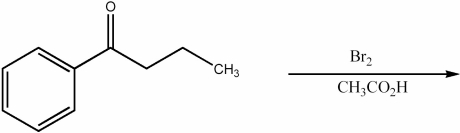


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
60
Which set of reagents would best accomplish the following transformation? 
A) Br2 / HBr
B) Br2 / PBr3
C) Br2 / NaOH
D) PBr3

A) Br2 / HBr
B) Br2 / PBr3
C) Br2 / NaOH
D) PBr3

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
61
What product results when pentanal is heated with sodium hydroxide?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
62
Provide the major organic product of the following reaction. 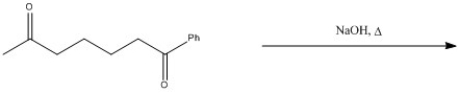


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
63
Draw a mechanism and provide the structure of the aldol product that results when 4-methylpentanal is heated with sodium hydroxide.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
64
What product results when an aldol is dehydrated?
A) conjugated alkyne
B) β-diketone
C) β-ketoester
D) α,β-unsaturated aldehyde
E) β,γ-unsaturated aldehyde
A) conjugated alkyne
B) β-diketone
C) β-ketoester
D) α,β-unsaturated aldehyde
E) β,γ-unsaturated aldehyde

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
65
Provide the structure of the intramolecular aldol condensation/dehydration product that results when 2,6-heptanedione is heated in base.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
66
In theory a poorly planned crossed aldol reaction can produce how many different products?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following could result from the dehydration of a self-aldol condensation product?
A) 4-methyl-3-penten-2-one
B) 4-methyl-4-penten-2-one
C) 4-methyl-5-hexen-2-one
D) 4-methyl-4-hexen-2-one
E) 3-methyl-4-penten-2-one
A) 4-methyl-3-penten-2-one
B) 4-methyl-4-penten-2-one
C) 4-methyl-5-hexen-2-one
D) 4-methyl-4-hexen-2-one
E) 3-methyl-4-penten-2-one

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
68
Predict the outcome of the following reaction. 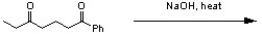
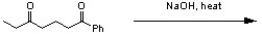

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
69
Provide the structure of the ester that would undergo self-condensation to yield the 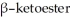
shown below.
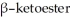
shown below.


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
70
What two molecules were condensed in an aldol reaction to produce 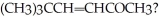
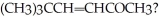

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
71
What two organic starting materials are required to produce cinnamaldehyde 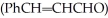
via a crossed aldol condensation followed by dehydration?
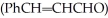
via a crossed aldol condensation followed by dehydration?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
72
Provide the structure of the Claisen product in the self condensation of methyl phenylacetate.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
73
What two molecules were condensed in an aldol reaction to produce 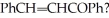
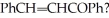

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
74
The following compound isolated from a marine sponge has been found to have anti-tubercular activity (Tet. Lett. 2007, 8851). Show how it may be constructed by an aldol reaction by drawing a structure of the starting material. 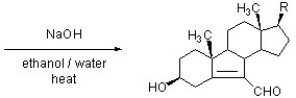


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
75
What crossed-aldol product results when propanal is heated in the presence of excess 2,2-dimethylpropanal and sodium hydroxide?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
76
Name the aldol produced when butanal is treated with NaOH.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
77
Provide the structure of the product which results from the base-catalyzed condensation followed by dehydration between benzophenone and propanal.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
78
Predict the pKa unit of the indicated proton within +/- 1 pKa unit. 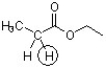
A) 5
B) 11
C) 19
D) 24

A) 5
B) 11
C) 19
D) 24

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
79
Provide the sequence of steps necessary to synthesize the compound shown below from cyclohexene. 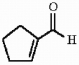


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
80
What crossed-aldol product results when butanal is heated in the presence of excess benzaldehyde and sodium hydroxide?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck


