Deck 20: Carboxylic Acids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/125
Play
Full screen (f)
Deck 20: Carboxylic Acids
1
Provide the name of the compound shown below. 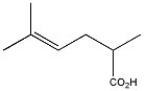

2,5-dimethyl-4-hexenoic acid or 2,5-dimethylhex-4-enoic acid
2
Provide the structure of 3,3-dimethylheptanoic acid.
CH3CH2CH2CH2C(CH3)2CH2CO2H
3
What is the common name for the following compound? 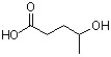
A) γ-hydroxyvaleric acid
B) γ-hydroxypentanoic acid
C) γ-hydroxy-γ-methylbutyric acid
D) δ-hydroxyvaleric acid

A) γ-hydroxyvaleric acid
B) γ-hydroxypentanoic acid
C) γ-hydroxy-γ-methylbutyric acid
D) δ-hydroxyvaleric acid
γ-hydroxyvaleric acid
4
Name the compound shown below. 


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
5
Carboxylic acids boil at considerably higher temperatures than do alcohols, ketones, or aldehydes of similar molecular weights. This is because they ________.
A) have a greater oxygen content
B) are more acidic
C) form stable hydrogen-bonded dimers
D) are hydrophobic
E) none of the above
A) have a greater oxygen content
B) are more acidic
C) form stable hydrogen-bonded dimers
D) are hydrophobic
E) none of the above

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
6
Provide the IUPAC name for the compound shown below. 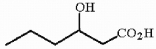


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
7
Provide the IUPAC name for the compound shown below. 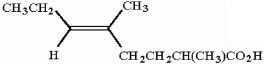


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
8
Provide the IUPAC name for the compound shown below. 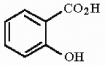


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
9
Name the compound shown below. 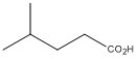


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
10
Draw an acetic acid dimer. Be sure to indicate the hydrogen bonds present.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
11
Provide the IUPAC name for HO2CCH2C(CH3)2CH2CH2CO2H.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
12
Provide the structure of glutaric acid.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
13
Provide the structure of pent-3-ynoic acid.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
14
Name the compound shown below. 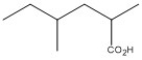


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
15
Provide the structure of 3-nitrophthalic acid.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
16
The common name for pentanedioic acid is ________.
A) pimelic acid
B) oxalic acid
C) glutaric acid
D) succinic acid
E) adipic acid
A) pimelic acid
B) oxalic acid
C) glutaric acid
D) succinic acid
E) adipic acid

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
17
Provide the structure of trans-1,3-cyclohexanedicarboxylic acid.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
18
Are carboxylic acids of more than 10 carbons more soluble in polar or nonpolar solvents?

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
19
The combination of a carbonyl group and a hydroxyl group on the same carbon atom is called a ________ group.
A) carbamate
B) carbonate
C) urethane
D) carboxyl
E) carboxylate
A) carbamate
B) carbonate
C) urethane
D) carboxyl
E) carboxylate

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
20
Provide the structure of succinic acid.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
21
What products result from the reaction of sodium propanoate with hydrobromic acid?

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
22
Show the deprotonation of benzoic acid by hydroxide and draw the important resonance structures of the carboxylate ion.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
23
An ether solution of PhCO2H (A), PhNH2 (B), and PhCH3 (C) is extracted with aqueous NaOH. The ether layer will contain what compound(s) after the extraction?
A) A + B
B) A + C
C) B + C
D) A + B + C
E) A only
A) A + B
B) A + C
C) B + C
D) A + B + C
E) A only

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
24
The pKa1 for oxalic acid (pKa = 1.27) is much lower than the pKa1 of glutaric acid (pKa = 4.35). Briefly explain why.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
25
What salt results from the reaction of benzoic acid with potassium hydroxide?

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
26
An unknown compound is insoluble in water but dissolves in an aqueous solution of sodium bicarbonate with a release of carbon dioxide bubbles. The compound is almost certainly ________.
A) a carboxylic acid
B) an amine
C) an aldehyde
D) an alkyl chloride
E) an alcohol
A) a carboxylic acid
B) an amine
C) an aldehyde
D) an alkyl chloride
E) an alcohol

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
27
The strongest dichlorobutanoic acid is ________.
A) 2,2-dichlorobutanoic acid
B) 2,3-dichlorobutanoic acid
C) 3,3-dichlorobutanoic acid
D) 3,4-dichlorobutanoic acid
E) 4,4-dichlorobutanoic acid
A) 2,2-dichlorobutanoic acid
B) 2,3-dichlorobutanoic acid
C) 3,3-dichlorobutanoic acid
D) 3,4-dichlorobutanoic acid
E) 4,4-dichlorobutanoic acid

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following sequences ranks the structures below in order of increasing
Acidity?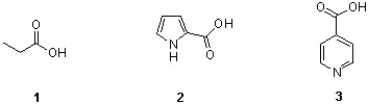
A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 2 < 1 < 3
Acidity?

A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 2 < 1 < 3

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
29
Using acid-base extractions, how might you purify a crude sample of benzoic acid?

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is the strongest acid?
A) chloroacetic acid
B) dichloroacetic acid
C) trichloroacetic acid
D) acetic acid
A) chloroacetic acid
B) dichloroacetic acid
C) trichloroacetic acid
D) acetic acid

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
31
In glutaric acid (HOOCCH2CH2CH2COOH), pKa1 is 4.35 while the pKa2 is 5.42. Why is the second carboxylic acid less acidic?

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
32
List the following weak acids in order of increasing acidity (from lowest to highest.). 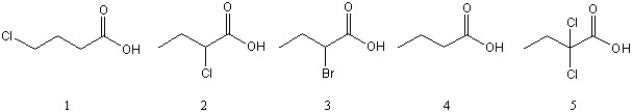
A) 4 < 3 < 2 < 1 < 5
B) 4 < 1 < 3 < 2 < 5
C) 5 < 2 < 3 < 1 < 4
D) 4 < 1 < 2 < 5 < 3
E) 1 < 2 < 4 < 3 < 5

A) 4 < 3 < 2 < 1 < 5
B) 4 < 1 < 3 < 2 < 5
C) 5 < 2 < 3 < 1 < 4
D) 4 < 1 < 2 < 5 < 3
E) 1 < 2 < 4 < 3 < 5

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is the strongest acid?
A) acetic acid
B) chloroacetic acid
C) bromoacetic acid
D) fluoroacetic acid
A) acetic acid
B) chloroacetic acid
C) bromoacetic acid
D) fluoroacetic acid

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is the strongest acid?
A) (CH3)2CHCO2H
B) CH3CH2CO2H
C) CH3OCH2CO2H
D) PhCH2CO2H
E) O2NCH2CO2H
A) (CH3)2CHCO2H
B) CH3CH2CO2H
C) CH3OCH2CO2H
D) PhCH2CO2H
E) O2NCH2CO2H

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
35
The 1H NMR spectrum of an unknown acid has the following peaks:
Δ (ppm) = 12.71 (1H, s), 8.04 (2H, d), 7.30 (2H, d), 2.41 (3H, s)
Which structure best fits this spectral information?
A)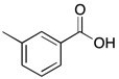
B)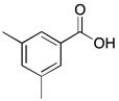
C)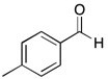
D)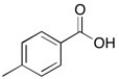
Δ (ppm) = 12.71 (1H, s), 8.04 (2H, d), 7.30 (2H, d), 2.41 (3H, s)
Which structure best fits this spectral information?
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
36
Why are the OH groups of carboxylic acids more acidic than alcohols?
A) resonance stabilization of the carboxylate ion
B) inductive electron donating by the carbonyl oxygen
C) reduced hydrogen bonding capacity
D) because they have lower pKa values
E) None of the above - carboxylic acids are not more acidic than alcohols.
A) resonance stabilization of the carboxylate ion
B) inductive electron donating by the carbonyl oxygen
C) reduced hydrogen bonding capacity
D) because they have lower pKa values
E) None of the above - carboxylic acids are not more acidic than alcohols.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following compounds is the strongest acid?
A) p-nitrobenzoic acid
B) p-bromobenzoic acid
C) m-methylbenzoic acid
D) m-methoxybenzoic acid
E) water
A) p-nitrobenzoic acid
B) p-bromobenzoic acid
C) m-methylbenzoic acid
D) m-methoxybenzoic acid
E) water

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
38
Provide the major organic product of the reaction shown below. 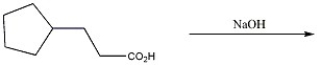


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
39
Name the salt formed from the reaction of acetic acid with ammonia.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
40
After completing the synthesis of 3-methylpentanoic acid, which of the following treatments will neutralize the mineral acids and facilitate the distribution of the organic acid from the organic layer to the aqueous extraction layer?
A) extraction with aqueous NaCl
B) extraction with ether
C) extraction with aqueous NaHCO3
D) extraction with water
E) extraction with dilute aqueous HCl
A) extraction with aqueous NaCl
B) extraction with ether
C) extraction with aqueous NaHCO3
D) extraction with water
E) extraction with dilute aqueous HCl

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
41
In the mass spectrum of 3-methylhexanoic acid, predict the mass of the fragment resulting from a McLafferty rearrangement.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
42
Carboxylic acids can be made from Grignards by treating the Grignard reagents with ________.
A) carbon monoxide
B) esters
C) aldehydes
D) diborane
E) carbon dioxide
A) carbon monoxide
B) esters
C) aldehydes
D) diborane
E) carbon dioxide

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
43
What two features are prominent in the infrared spectrum of a carboxylic acid?

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
44
How does the O-H stretch in the IR spectrum of a carboxylic acid differ from the O-H stretch of an alcohol?

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
45
In a carboxylic acid, the hybridization of the C is ________ and the OCO bond angle is ________.
A) sp2; exactly 120°
B) sp2; greater than 120°
C) sp3; 109.5°
D) sp3; less than 120°
A) sp2; exactly 120°
B) sp2; greater than 120°
C) sp3; 109.5°
D) sp3; less than 120°

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
46
Provide the major organic product of the following reaction. 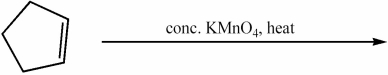


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
47
Provide the major organic product of the following reaction. 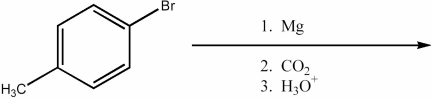
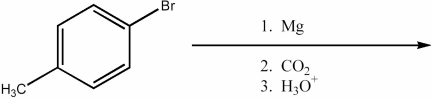

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
48
2-Phenylethanol yields what acid upon treatment with cold chromic acid?

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
49
Where would one expect to find the 1H NMR signal for the carboxyl group's hydrogen in propanoic acid?
A) δ 4.1 - 5.6 ppm
B) δ 10 - 13 ppm
C) δ 8 - 9 ppm
D) δ 6.1 - 7.8 ppm
E) δ 9.5 - 10 ppm
A) δ 4.1 - 5.6 ppm
B) δ 10 - 13 ppm
C) δ 8 - 9 ppm
D) δ 6.1 - 7.8 ppm
E) δ 9.5 - 10 ppm

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
50
An acid which could not be prepared by the reaction of an organic halide with cyanide ion followed by acid hydrolysis of the nitrile is ________.
A) propanoic acid
B) phenylacetic acid
C) acetic acid
D) (CH3)3CCO2H
E) CH3(CH2)14CO2H
A) propanoic acid
B) phenylacetic acid
C) acetic acid
D) (CH3)3CCO2H
E) CH3(CH2)14CO2H

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following must be converted into a carboxylic acid through nitrile hydrolysis rather than through a Grignard synthesis with Mg/ether followed with dry CO2 and work-up with H3O+? 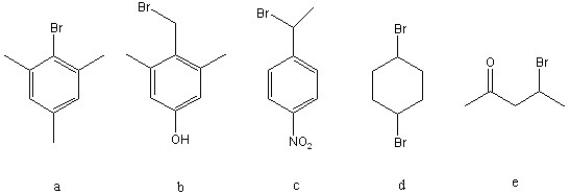


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
52
Deduce a reasonable structure for the compound which exhibits the following spectroscopic data.
C5H9ClO2: IR: 2700-3400 cm-1 (broad), 1710 cm-1; 1H NMR (ppm): 1.40 (6H, singlet), 3.60 (2H, singlet), 10.1 (1H, singlet) ppm.
C5H9ClO2: IR: 2700-3400 cm-1 (broad), 1710 cm-1; 1H NMR (ppm): 1.40 (6H, singlet), 3.60 (2H, singlet), 10.1 (1H, singlet) ppm.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
53
In the mass spectrum of pentanoic acid, the base peak occurs at m/z ________.
A) 102
B) 101
C) 85
D) 73
E) 60
A) 102
B) 101
C) 85
D) 73
E) 60

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
54
Starting with any alkene, how could you make pimelic acid (HOOC(CH2)5COOH)?

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
55
What compound is produced when cyclohexene is treated with concentrated KMnO4?
A) hexanoic acid
B) adipic acid
C) cyclohexanecarboxylic acid
D) benzoic acid
E) succinic acid
A) hexanoic acid
B) adipic acid
C) cyclohexanecarboxylic acid
D) benzoic acid
E) succinic acid

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
56
1-Hexanol reacts with chromic acid to yield what product?

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
57
What compound is produced when (CH3)2CHCH2Br is subjected to the following sequence of steps?
1) Mg, Et2O 2. CO2
A) 2-methylpropanoic acid
B) 3-methylpropanoic acid
C) 2-methylbutanoic acid
D) 3-methylbutanoic acid
E) 2-methylhexanoic acid
1) Mg, Et2O 2. CO2
A) 2-methylpropanoic acid
B) 3-methylpropanoic acid
C) 2-methylbutanoic acid
D) 3-methylbutanoic acid
E) 2-methylhexanoic acid

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
58
An acid which could not be prepared from an organic halide by carboxylation of the Grignard reagent is ________.
A) benzoic acid
B) 2,2-dimethylpropanoic acid
C) propanoic acid
D) 4-oxocyclohexanecarboxylic acid
E) 2-methylbutanoic acid
A) benzoic acid
B) 2,2-dimethylpropanoic acid
C) propanoic acid
D) 4-oxocyclohexanecarboxylic acid
E) 2-methylbutanoic acid

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
59
Provide the major organic product of the following reaction. 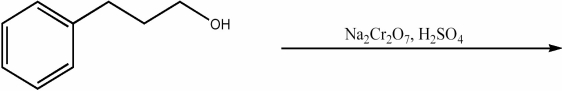

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following statements is true?
A) The carbonyl carbon in a carboxylic acid does not give a 13C signal in a 13C-NMR spectrum.
B) The carbonyl carbon in a carboxylic acid gives a 13C signal in the same region as a carbonyl carbon from a ketone or aldehyde - in the range of 200 ppm.
C) The carbonyl carbon of a carboxylic acid splits a proton signal into a doublet in an H-NMR spectrum.
D) The carbonyl carbon in a carboxylic acid gives a 13C signal in the same region as a carbonyl carbon from an ester or amide in the range of 150 to 180 ppm.
E) The carbonyl carbon in a carboxylic acid cannot be distinguished from an aromatic carbon because they both give signals in the range of 110 to 130 ppm.
A) The carbonyl carbon in a carboxylic acid does not give a 13C signal in a 13C-NMR spectrum.
B) The carbonyl carbon in a carboxylic acid gives a 13C signal in the same region as a carbonyl carbon from a ketone or aldehyde - in the range of 200 ppm.
C) The carbonyl carbon of a carboxylic acid splits a proton signal into a doublet in an H-NMR spectrum.
D) The carbonyl carbon in a carboxylic acid gives a 13C signal in the same region as a carbonyl carbon from an ester or amide in the range of 150 to 180 ppm.
E) The carbonyl carbon in a carboxylic acid cannot be distinguished from an aromatic carbon because they both give signals in the range of 110 to 130 ppm.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
61
Propose a reasonable synthetic route to prepare cyclohexylacetic acid from methylenecyclohexane.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
62
Draw a Fischer projection of the product which results when (R)-2-bromobutane is treated with the following sequence of reagents: 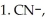
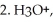
and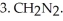
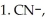
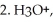
and
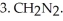

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
63
Provide the major organic product of the following reaction. 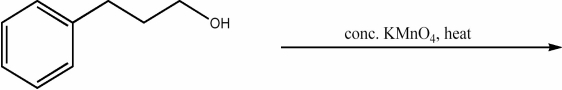
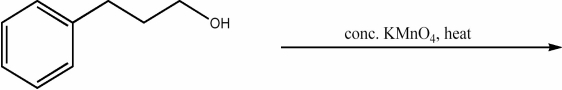

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
64
Using the carboxylic acid below as your only carbon source, how would you make the given amide? 


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
65
Provide the major organic product of the reaction shown below. 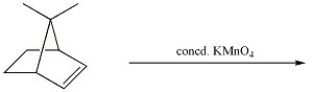


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
66
Provide the major organic product of the following reaction. 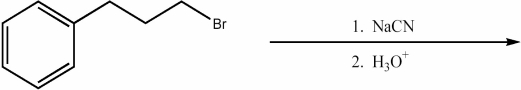


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
67
Suggest a sequence of synthetic steps through which phenylacetic acid can be prepared from toluene via phenylacetonitrile.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
68
Suggest a sequence of synthetic steps through which p-toluic acid can be prepared from toluene.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
69
What two alkenes, which contain only one double bond, yield exclusively propanoic acid upon oxidation with hot concentrated KMnO4?

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
70
Provide the major organic product of the reaction shown below. 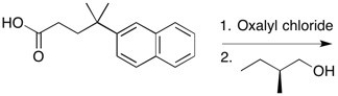


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
71
Hept-3-yne yields what acids upon treatment with concentrated permanganate of ozone followed by water?

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
72
Suggest a sequence of synthetic steps through which phenylacetic acid can be prepared from toluene and in which Grignard chemistry is employed.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
73
Provide the major organic product of the reaction shown below. 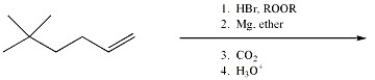
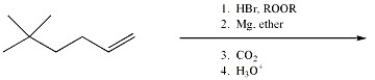

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
74
Provide the sequence of reagents needed to accomplish the conversion below. 


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
75
Show how you could make the acid shown below starting with any alcohol: 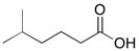


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
76
An unknown alkyne was subjected to concentrated KMnO4 and only one product was isolated. The spectral information for 1H NMR of the product is given below.
δ (ppm) = 11.0 (1H, broad s), 2.39 (2H, q), 1.16 (3H, t)
What is the structure of the starting alkyne?
δ (ppm) = 11.0 (1H, broad s), 2.39 (2H, q), 1.16 (3H, t)
What is the structure of the starting alkyne?

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
77
Provide the major organic product of the following reaction sequence. 


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
78
Show how you could convert benzene to benzoic acid.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
79
Which sequence of steps below describes the best synthesis of 5-oxohexanoic acid starting with 1-methylcyclopentan-1-ol?
A) 1. Conc. KMnO4
2) Dry gaseous HBr
3) mg/ether
4) CO2
B) 1. H2SO4 and heat
2) Conc. KMnO4
C) 1. Conc. KMnO4
2) CH3MgBr/ ether
3. H3O+
D) 1. H2SO4 and heat
2) O3
3) (CH3)2S
4) PCC
E) 1. H2SO4 and heat
2) Conc. KMnO4
3) LiAlH4
4) H3O+
A) 1. Conc. KMnO4
2) Dry gaseous HBr
3) mg/ether
4) CO2
B) 1. H2SO4 and heat
2) Conc. KMnO4
C) 1. Conc. KMnO4
2) CH3MgBr/ ether
3. H3O+
D) 1. H2SO4 and heat
2) O3
3) (CH3)2S
4) PCC
E) 1. H2SO4 and heat
2) Conc. KMnO4
3) LiAlH4
4) H3O+

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
80
Provide the major organic product of the following reaction sequence. 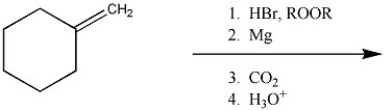
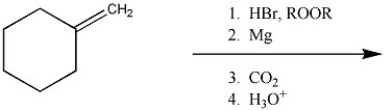

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck


