Deck 21: Carboxylic Acid Derivatives
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/130
Play
Full screen (f)
Deck 21: Carboxylic Acid Derivatives
1
Provide the IUPAC name of the following compound. 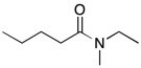

N-ethyl-N-methylpentanamide
2
N-Methylacetamide is an example of ________.
A) a primary amide
B) a secondary amide
C) a tertiary amide
D) an N, N-disubstituted amide
E) an imine
A) a primary amide
B) a secondary amide
C) a tertiary amide
D) an N, N-disubstituted amide
E) an imine
a secondary amide
3
Provide the structure of γ-butyrolactam.
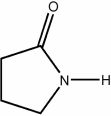
4
Provide the structure of (R)-4-ethoxypentanenitrile.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
5
Provide the structure of propanoic anhydride.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following structures are carboxylic acid derivatives? 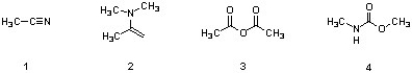


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
7
Provide the proper IUPAC name for the compound below. 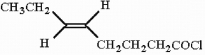


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
8
Provide the proper IUPAC name for ClCH2CH2CONHCH3.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
9
Cyclic amides are called ________.
A) lactones
B) lactams
C) aminals
D) animals
E) imines
A) lactones
B) lactams
C) aminals
D) animals
E) imines

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
10
Provide the proper IUPAC name for the compound below. 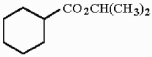


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
11
Cyclic esters are called ________.
A) lactones
B) lactams
C) lacrimals
D) imides
E) enamines
A) lactones
B) lactams
C) lacrimals
D) imides
E) enamines

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
12
Provide the structure of cyclohexyl formate.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
13
Provide the structure of trifluoroacetic anhydride.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
14
Provide the IUPAC name of the following compound. 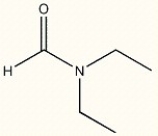


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
15
Provide the proper IUPAC name for the compound below. 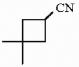


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
16
Polycaprolactone (shown below) is a biodegradable polymer that can be synthesized from a lactone. Suggest a lactone monomer that could be used to make this polymer.
-[OCO(CH2)5-]n-
-[OCO(CH2)5-]n-

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
17
Provide the name of the compound shown below. 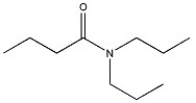


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
18
Provide the name of the compound shown below. 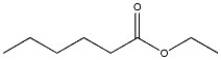


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
19
Amides are less basic than amines because ________.
A) the carbonyl group donates electrons by resonance
B) the carbonyl group withdraws electrons by resonance
C) the nitrogen does not have a lone pair of electrons
D) the nitrogen has a full positive charge
E) amides do not contain nitrogen
A) the carbonyl group donates electrons by resonance
B) the carbonyl group withdraws electrons by resonance
C) the nitrogen does not have a lone pair of electrons
D) the nitrogen has a full positive charge
E) amides do not contain nitrogen

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
20
Provide the structure of o-bromobenzoyl chloride.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
21
List the following five compounds, ethanamide, 1-propanol, methyl formate, acetic acid, and propanenitrile, in order of increasing boiling point. Start with the lowest boiling compound.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
22
How could you tell the two compounds apart using IR spectroscopy? 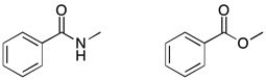


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
23
What is the correct IUPAC name for the following compound? 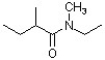
A) N-ethyl-2,N-dimethylbutanamide
B) N-ethyl-N-methylisobutyramide
C) 2,N-dimethyl-N-ethylbutanamide
D) 1-(ethylmethylamino)-2-methylbutanamide

A) N-ethyl-2,N-dimethylbutanamide
B) N-ethyl-N-methylisobutyramide
C) 2,N-dimethyl-N-ethylbutanamide
D) 1-(ethylmethylamino)-2-methylbutanamide

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
24
Provide the name of the compound shown below. 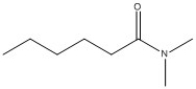


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
25
Provide the structure of N,N-diethylbutanamide.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
26
Identify the correct IUPAC systematic name for the structure below. 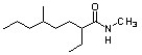
A) N-methyl-2-ethyl-5-methyloctanamide
B) 6,N-dimethylnonane-3-carboxamide
C) 5,N-dimethyl-2-ethyloctanamide
D) 2-ethyl-5,N-dimethyloctanamide

A) N-methyl-2-ethyl-5-methyloctanamide
B) 6,N-dimethylnonane-3-carboxamide
C) 5,N-dimethyl-2-ethyloctanamide
D) 2-ethyl-5,N-dimethyloctanamide

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
27
While the carbonyl stretching frequency for simple aldeydes, ketones, and carboxylic acids is about 1710 cm-1, the carbonyl stretching frequency for esters is about ________.
A) 1660 cm-1
B) 1700 cm-1
C) 1735 cm-1
D) 1800 cm-1
E) 2200 cm-1
A) 1660 cm-1
B) 1700 cm-1
C) 1735 cm-1
D) 1800 cm-1
E) 2200 cm-1

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
28
What is the common name for the following carboxylic acid derivative? 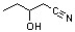
A) β-hydroxybutyronitrile
B) 1-cyano-2-butanol
C) β-hydroxyvaleronitrile
D) γ-hydroxyvaleronitrile
E) 2-hydroxypentane nitrile

A) β-hydroxybutyronitrile
B) 1-cyano-2-butanol
C) β-hydroxyvaleronitrile
D) γ-hydroxyvaleronitrile
E) 2-hydroxypentane nitrile

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
29
Arrange the following compounds in order of increasing boiling point: acetic acid, propanenitrile, butane, and acetamide.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
30
Arrange the following three amides in order of increasing boiling point: propanamide, N-methylacetamide, and N,N-dimethylformamide.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
31
Provide the proper IUPAC name for NCCH2CH2CH2CH2CO2CH3.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
32
Provide the name of CH3O2CH2CH2CH2CH3.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following are strongly hydrogen bonded in the liquid phase?
A) nitriles
B) esters
C) secondary amides
D) tertiary amides
E) acid chlorides
A) nitriles
B) esters
C) secondary amides
D) tertiary amides
E) acid chlorides

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
34
Provide the structure of 2-bromo-2-methylbutanoyl chloride.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
35
In 1H NMR, the chemical shifts for protons α to a carbonyl group ________.
A) are similar for all acid derivatives
B) are farthest downfield for carboxylic acids
C) are farther downfield than those α to a nitrile
D) fall between δ 3.1 and δ 4.2 (ppm)
E) fall between δ 1.3 and δ 1.8 (ppm)
A) are similar for all acid derivatives
B) are farthest downfield for carboxylic acids
C) are farther downfield than those α to a nitrile
D) fall between δ 3.1 and δ 4.2 (ppm)
E) fall between δ 1.3 and δ 1.8 (ppm)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
36
While the carbonyl stretching frequency for simple aldehydes, ketones, and carboxylic acids is about 1710 cm-1, the carbonyl stretching frequency for acid chlorides is about ________.
A) 1700 cm-1
B) 1735 cm-1
C) 1800 cm-1
D) 1660 cm-1
E) 2200 cm-1
A) 1700 cm-1
B) 1735 cm-1
C) 1800 cm-1
D) 1660 cm-1
E) 2200 cm-1

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
37
While the carbonyl stretching frequency for simple aldehydes, ketones, and carboxylic acids is about 1710 cm-1, the carbonyl stretching frequency for amides is about ________.
A) 1700 cm-1
B) 1735 cm-1
C) 1800 cm-1
D) 1660 cm-1
E) 2200 cm-1
A) 1700 cm-1
B) 1735 cm-1
C) 1800 cm-1
D) 1660 cm-1
E) 2200 cm-1

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
38
Provide the IUPAC name for HCONHCH2CH2CH3.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
39
Provide the structure of 3-oxobutanenitrile.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
40
Provide the name of the compound shown below. 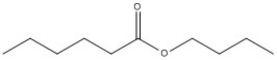


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
41
Name the compound which results when δ-valerolactone is heated in a large excess of methanol in the presence of catalytic acid.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is the most reactive carboxylic acid derivative?
A) ester
B) anhydride
C) nitrile
D) acid chloride
E) amide
A) ester
B) anhydride
C) nitrile
D) acid chloride
E) amide

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
43
Draw the product resulting from the following reaction. Indicate any relevant stereochemistry. 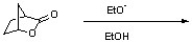


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
44
Using 1H NMR, how would you tell the two esters apart? 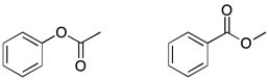


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
45
Provide a detailed, stepwise mechanism for the base-mediated transesterification of methyl benzoate with sodium ethoxide.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
46
Provide a detailed, stepwise mechanism for the acid-catalyzed transesterification of ethyl acetate with n-propanol.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
47
Arrange the carboxylic acid derivatives below in order of increasing reactivity towards nucleophilic acyl substitution. 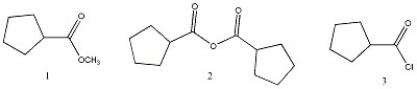
A) 1 < 2 <3
B) 1 < 3 < 2
C) 2 < 1 < 3
D) 2 < 3 < 1
E) 3 < 2 < 1

A) 1 < 2 <3
B) 1 < 3 < 2
C) 2 < 1 < 3
D) 2 < 3 < 1
E) 3 < 2 < 1

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
48
The most characteristic IR stretch of butanenitrile occurs at approximately what wavenumber?
A) 3500 cm-1
B) 3100 cm-1
C) 2600 cm-1
D) 2200 cm-1
E) 1750 cm-1
A) 3500 cm-1
B) 3100 cm-1
C) 2600 cm-1
D) 2200 cm-1
E) 1750 cm-1

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
49
Provide an arrow pushing mechanism for the following reaction. Show all intermediate structures and formal charges. You do not need to show resonance structures. 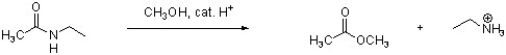
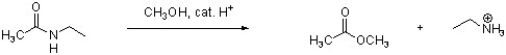

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
50
In nucleophilic acyl substitution ________.
A) protonation of the carbonyl is followed immediately by loss of the leaving group
B) loss of the leaving group is followed by rearrangement of the carbocation
C) addition to the carbonyl by a nucleophile is followed by loss of the leaving group
D) ester hydrolysis is followed by deprotonation
E) an SN2 reaction occurs
A) protonation of the carbonyl is followed immediately by loss of the leaving group
B) loss of the leaving group is followed by rearrangement of the carbocation
C) addition to the carbonyl by a nucleophile is followed by loss of the leaving group
D) ester hydrolysis is followed by deprotonation
E) an SN2 reaction occurs

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
51
Explain why alkoxides act as leaving groups in nucleophilic acyl substitutions but not in SN2 reactions.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
52
Explain while this reaction won't proceed as it is written in the forward direction. 


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
53
Describe, in short phrases, the three step mechanism by which hexanoyl chloride reacts with dimethylamine.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
54
Shown below is the structure of Lactimidomycin, a potent inhibitor of cell migration, proliferation and protein synthesis. Identify the carboxylic acid derivative/s in the structure of Lactimidomycin. 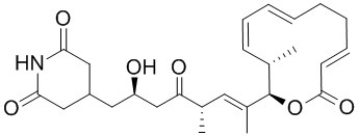
A) Ester
B) Anhydride
C) Nitrile
D) Imine

A) Ester
B) Anhydride
C) Nitrile
D) Imine

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
55
Name the compound which results when propyl benzoate is heated in a large excess of ethanol in the presence of catalytic acid.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
56
Predict the major product of the following reaction. 


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
57
List the following four carboxylic acid derivatives, ester, anhydride, amide, and acid halide, in order of increasing reactivity in nucleophilic acyl substitution reactions. Start with the least reactive derivative.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following reactions is favorable, in the direction indicated, under fairly "normal" laboratory conditions?
A)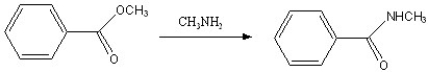
B)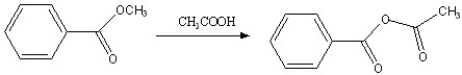
C)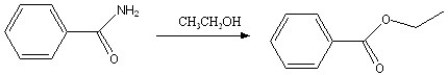
D)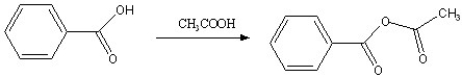
E)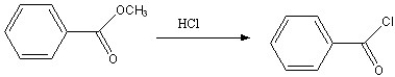
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
59
A correct order of reactivity of acid derivatives towards nucleophilic attack is ________.
A) esters > acid anhydrides > amides
B) anhydrides > amides > esters
C) carboxylates > esters > amides
D) anhydrides > acids > acid chlorides
E) anhydrides > amides > carboxylates
A) esters > acid anhydrides > amides
B) anhydrides > amides > esters
C) carboxylates > esters > amides
D) anhydrides > acids > acid chlorides
E) anhydrides > amides > carboxylates

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
60
What compound results when ethyl benzoate is stirred in methanol containing a trace of HCl and by what name is this process known?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
61
Distamycin and derivatives have exhibited antiviral, antibiotic, and antitumor activity by binding to the minor groove of DNA (J. Med. Chem. 2004, 2133). Place a line through each bond of distamycin that would be cleaved by acid hydrolysis. 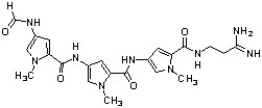


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following are intermediates in the acid hydrolysis of an amide? 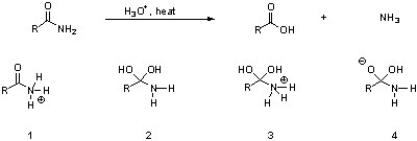
A) 1
B) 2
C) 2 & 3
D) 4
E) 1, 2, & 3

A) 1
B) 2
C) 2 & 3
D) 4
E) 1, 2, & 3

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
63
Acids can be converted to primary amines by ________.
A) conversion to the nitrile followed by treatment with LiAlH4
B) conversion to the diazonium salt followed by treatment with NaBH4
C) conversion to the primary amide followed by treatment with LiAlH4
D) both A and B
E) both A and C
A) conversion to the nitrile followed by treatment with LiAlH4
B) conversion to the diazonium salt followed by treatment with NaBH4
C) conversion to the primary amide followed by treatment with LiAlH4
D) both A and B
E) both A and C

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
64
Acids can be reduced to aldehydes by ________.
A) treatment with LiAlH4
B) conversion to the acid chloride followed by treatment with LiAlH[OC(CH3)3]3
C) conversion to the amide followed by treatment with NaBH4
D) conversion to the ester followed by treatment with LiAlH4
E) conversion to the anhydride followed by treatment with Mg and H3O+
A) treatment with LiAlH4
B) conversion to the acid chloride followed by treatment with LiAlH[OC(CH3)3]3
C) conversion to the amide followed by treatment with NaBH4
D) conversion to the ester followed by treatment with LiAlH4
E) conversion to the anhydride followed by treatment with Mg and H3O+

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
65
Typically, amides will hydrolyze under ________ conditions than esters.
A) stronger
B) more dilute
C) milder
D) less vigorous
E) more saline
A) stronger
B) more dilute
C) milder
D) less vigorous
E) more saline

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
66
Two equivalents of methyl magnesium bromide will add to ________.
A) lactones
B) aldehydes
C) ketones
D) imines
E) secondary amines
A) lactones
B) aldehydes
C) ketones
D) imines
E) secondary amines

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
67
Provide the major organic product in the reaction shown below. 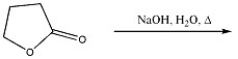
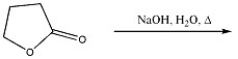

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
68
Predict the major product of the following reaction. 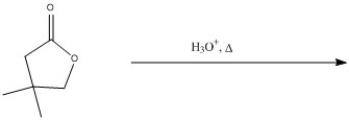


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
69
The hydrolysis of esters in base is called ________.
A) the Fischer esterification
B) the Hunsdiecker reaction
C) the Dieckmann condensation
D) transesterification
E) saponification
A) the Fischer esterification
B) the Hunsdiecker reaction
C) the Dieckmann condensation
D) transesterification
E) saponification

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
70
Predict the major product of the following reaction. 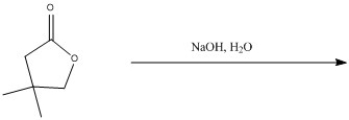


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
71
Predict the major product of the following reaction. 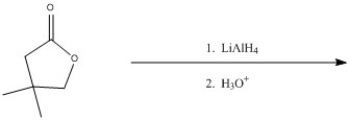


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
72
Provide the major organic product in the reaction shown below. 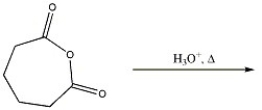


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following compounds is(are) hydrolyzed to butanoic acid upon heating in 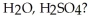
A) ethyl butanoate
B) butyl acetate
C) N-methylbutanamide
D) both A and B
E) both A and C
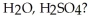
A) ethyl butanoate
B) butyl acetate
C) N-methylbutanamide
D) both A and B
E) both A and C

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
74
Predict the major product of the following reaction. 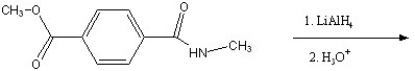


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
75
What compound is produced when N,N-dimethylpropanamide is treated with LiAlH4?
A) CH3CH2CONH2
B) CH3CH2CH2NH2
C) CH3CH2CH2OH
D) CH3CH2CH2N(CH3)2
E) CH3CH2N(CH3)2
A) CH3CH2CONH2
B) CH3CH2CH2NH2
C) CH3CH2CH2OH
D) CH3CH2CH2N(CH3)2
E) CH3CH2N(CH3)2

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
76
Which sequence ranks the following carboxylic acid derivatives in order of increasing rate of hydrolysis? 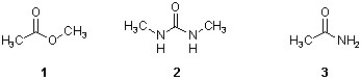
A) 2 < 3 < 1
B) 3 < 2 < 1
C) 2 < 1 < 3
D) 1 < 3 < 2

A) 2 < 3 < 1
B) 3 < 2 < 1
C) 2 < 1 < 3
D) 1 < 3 < 2

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
77
Provide the major organic product in the reaction of CH3CH2CONH2 with hot aqueous acid.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
78
Predict the major organic product in the following reaction. 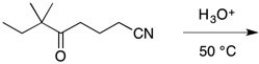


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
79
The hydrolysis of esters, amides, and nitriles ________.
A) can be carried out under acidic or basic conditions
B) must be acid-catalyzed
C) must be base-catalyzed
D) should be carried out at pH 7.0 for optimum efficiency
E) is not pH dependent
A) can be carried out under acidic or basic conditions
B) must be acid-catalyzed
C) must be base-catalyzed
D) should be carried out at pH 7.0 for optimum efficiency
E) is not pH dependent

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
80
Lithium aluminum hydride reduces carboxylic acids, acid chlorides, and esters to ________.
A) aldehydes
B) primary alcohols
C) secondary alcohols
D) tertiary alcohols
E) ketones
A) aldehydes
B) primary alcohols
C) secondary alcohols
D) tertiary alcohols
E) ketones

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck


