Deck 12: Infrared Spectroscopy and Mass Spectrometry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/121
Play
Full screen (f)
Deck 12: Infrared Spectroscopy and Mass Spectrometry
1
Absorption of what type of electromagnetic radiation results in transitions among allowed rotational motions?
A) X-rays
B) radio waves
C) microwaves
D) ultraviolet light
E) infrared light
A) X-rays
B) radio waves
C) microwaves
D) ultraviolet light
E) infrared light
microwaves
2
In what units are frequency values typically given?
Hertz or cycles/sec or sec-1
3
In which unit is frequency measured?
A) joule
B) Kcal
C) hertz
D) gauss
A) joule
B) Kcal
C) hertz
D) gauss
hertz
4
Absorption of what type of electromagnetic radiation results in electronic transitions?
A) X-rays
B) radio waves
C) microwaves
D) ultraviolet light
E) infrared light
A) X-rays
B) radio waves
C) microwaves
D) ultraviolet light
E) infrared light

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
5
Which region of the electromagnetic spectrum, IR or X-ray, is characterized by waves of lower frequency?

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
6
Which region of the electromagnetic spectrum, radio or visible, is characterized by waves of shorter wavelength?

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
7
Which sequence correctly ranks the regions of the electromagnetic spectrum in order of increasing energy?
1) infrared 2) ultraviolet 3) radio wave
A) 3 < 1 < 2
B) 3 < 2 < 1
C) 2 < 1 < 3
D) 1 < 3 < 2
1) infrared 2) ultraviolet 3) radio wave
A) 3 < 1 < 2
B) 3 < 2 < 1
C) 2 < 1 < 3
D) 1 < 3 < 2

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
8
Absorption of what type of electromagnetic radiation results in ionization?
A) X-rays
B) radio waves
C) microwaves
D) ultraviolet light
E) infrared light
A) X-rays
B) radio waves
C) microwaves
D) ultraviolet light
E) infrared light

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following equations is equal to frequency?
A) hλ
B) c/λ
C) hc/λ
D) 1/λ
A) hλ
B) c/λ
C) hc/λ
D) 1/λ

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
10
The wavelength and frequency of a given wave of electromagnetic radiation are ________ proportional.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
11
The energy of a photon is ________ proportional to its wavelength.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
12
Which region of the electromagnetic spectrum, IR or UV, contains photons of the higher energy?

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
13
Arrange the following regions of the electromagnetic spectrum in order of increasing wavelength: X ray, radio, UV, and IR.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
14
The energy of a photon is ________ proportional to its frequency.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
15
Absorption ________ is the measurement of the amount of light absorbed by a compound as a function of the wavelength of light.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
16
Absorption of what type of electromagnetic radiation results in transitions among allowed nuclear magnetic spin states?
A) X-rays
B) radio waves
C) microwaves
D) ultraviolet light
E) infrared light
A) X-rays
B) radio waves
C) microwaves
D) ultraviolet light
E) infrared light

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
17
Provide an equation which relates the energy of a photon to its wavelength.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
18
Which has the higher speed in a vacuum, ultraviolet or infrared light?

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
19
Arrange the following regions of the electromagnetic spectrum in order of increasing energy: microwave, UV, visible, and IR.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
20
Which bond in the structure below would give rise to the highest absorption frequency in an IR spectrum? 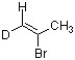
A) C sp2-H
B) C sp3-H
C) C-D
D) C=C

A) C sp2-H
B) C sp3-H
C) C-D
D) C=C

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
21
How could IR spectroscopy be used to distinguish between the following pair of compounds?
CH2

CHCH2CH(CH3)2 and CH3CH2CH2CH(CH3)2
CH2

CHCH2CH(CH3)2 and CH3CH2CH2CH(CH3)2

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
22
Which has a lower characteristic stretching frequency, the C-H or C-D bond? Explain briefly.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
23
An infrared wavelength of 4.48μm is equivalent to a wavenumber of ________ cm-1.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
24
Could pentane and heptane easily be distinguished by IR spectroscopy?

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following factors influences the intensity of a peak in the IR spectrum of a molecule?
A) mass of atoms
B) dipole moment
C) bond strength
D) bond length
A) mass of atoms
B) dipole moment
C) bond strength
D) bond length

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
26
In an IR spectrometer, the ________ uses prisms or diffraction gratings to allow only one frequency of light to enter the detector at a time.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
27
An infrared wavelength of 5.81μm is equivalent to a wavenumber of ________ cm-1.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
28
What wavenumber corresponds to the wavelength of 4 μm?

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the indicated bonds would be expected to have IR active frequencies? 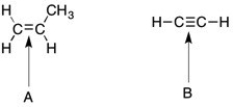
A) A only
B) B only
C) Both A and B
D) Neither A nor B
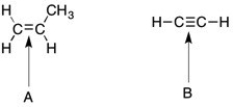
A) A only
B) B only
C) Both A and B
D) Neither A nor B

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
30
At approximately what wavenumber does one expect to find the carbon-carbon triple bond stretch in the IR spectrum of an alkyne?

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
31
What wavelength in mm is equivalent to a wavenumber of 1750 cm-1?

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
32
Absorption of what type of electromagnetic radiation results in transitions among allowed vibrational motions?
A) X-rays
B) radio waves
C) microwaves
D) ultraviolet light
E) infrared light
A) X-rays
B) radio waves
C) microwaves
D) ultraviolet light
E) infrared light

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
33
A nonlinear molecule with n atoms generally has ________ fundamental vibrational modes.
A) 2n
B) 2n - 2
C) 3n
D) 3n - 3
E) 3n - 6
A) 2n
B) 2n - 2
C) 3n
D) 3n - 3
E) 3n - 6

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
34
The frequency of the stretching vibration of a bond in IR spectroscopy depends on what two quantities?
A) the stiffness of the bond and the electronegativity of the atoms
B) the electronegativity of the atoms and the nuclear charges of the atoms
C) the masses of the atoms and the stiffness of the bond
D) the nuclear charges of the atoms and the atomic radii
E) the electronegativity of the atoms and the masses of the atoms
A) the stiffness of the bond and the electronegativity of the atoms
B) the electronegativity of the atoms and the nuclear charges of the atoms
C) the masses of the atoms and the stiffness of the bond
D) the nuclear charges of the atoms and the atomic radii
E) the electronegativity of the atoms and the masses of the atoms

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
35
In order for a vibration mode to be observable in the IR, the vibration must change the ________ of the molecule.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
36
Ethyne (HC  CH) does not show IR absorption in the region 2000-2500 cm-1 because ________.
CH) does not show IR absorption in the region 2000-2500 cm-1 because ________.
A) C-H stretches occur at lower energies
B) C C stretches occur at about 1640 cm-1
C stretches occur at about 1640 cm-1
C) there is no change in the dipole moment when the C C bond in ethyne stretches
C bond in ethyne stretches
D) there is a change in the dipole moment when the C C bond in ethyne stretches
C bond in ethyne stretches
 CH) does not show IR absorption in the region 2000-2500 cm-1 because ________.
CH) does not show IR absorption in the region 2000-2500 cm-1 because ________.A) C-H stretches occur at lower energies
B) C
 C stretches occur at about 1640 cm-1
C stretches occur at about 1640 cm-1C) there is no change in the dipole moment when the C
 C bond in ethyne stretches
C bond in ethyne stretchesD) there is a change in the dipole moment when the C
 C bond in ethyne stretches
C bond in ethyne stretches
Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
37
The region of the IR spectrum which generally contains the complex bending vibrations 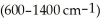
is called the ________ region of the spectrum.
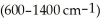
is called the ________ region of the spectrum.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
38
In IR spectroscopy, the C-O bond has a ________ frequency than the C-N bond because ________.
A) higher; an O atom has more mass than an N atom
B) lower; an O atom has more mass than an N atom
C) higher; an O atom has more electronegativity than an N atom
D) lower; an O atom has more electronegativity than an N atom
E) higher; an O atom has an even number of neutrons
A) higher; an O atom has more mass than an N atom
B) lower; an O atom has more mass than an N atom
C) higher; an O atom has more electronegativity than an N atom
D) lower; an O atom has more electronegativity than an N atom
E) higher; an O atom has an even number of neutrons

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
39
Which has a lower characteristic stretching frequency, the C 
O bond or the C-O bond? Explain briefly.

O bond or the C-O bond? Explain briefly.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following stretches tends to be the least intense?
A) O-H (alcohol)
B) O-H (carboxylic acid)
C) C-H
D) C=O
E) C=C
A) O-H (alcohol)
B) O-H (carboxylic acid)
C) C-H
D) C=O
E) C=C

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following has a C-H stretch that occurs at the lowest stretching frequency?
A) hexane
B) hex-1-ene
C) (E)-hex-2-ene
D) hex-1-yne
E) hex-2-yne
A) hexane
B) hex-1-ene
C) (E)-hex-2-ene
D) hex-1-yne
E) hex-2-yne

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
42
The IR spectrum of a sample contains absorptions at 3050, 2950, and 1620 cm-1. To what class of organic compound does this sample most likely belong?
A) alkane
B) alkene
C) alkyne
D) ester
E) alcohol
A) alkane
B) alkene
C) alkyne
D) ester
E) alcohol

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
43
Which compound has an IR absorption with a broad peak and a single spike at 3300 cm-1?
A) (CH3CH2)2NH
B) CH3CH2CH2OH
C) CH3CH2CN
D) (CH3CH2)2O
E) CH3CH=CHCH3
A) (CH3CH2)2NH
B) CH3CH2CH2OH
C) CH3CH2CN
D) (CH3CH2)2O
E) CH3CH=CHCH3

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
44
Which compound has a strong and broad IR absorption centered at 3400 cm-1?
A) 1-butanol
B) octane
C) diethyl ether
D) 2-hexyne
E) benzene
A) 1-butanol
B) octane
C) diethyl ether
D) 2-hexyne
E) benzene

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
45
Which compound would be expected to show intense IR absorption at 1715 cm-1?
A) (CH3)2CHNH2
B) hex-1-yne
C) 2-methylhexane
D) (CH3)2CHCO2H
A) (CH3)2CHNH2
B) hex-1-yne
C) 2-methylhexane
D) (CH3)2CHCO2H

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following would not have a C-H stretch at about 3050 cm-1?
A) 1-pentene
B) 2-pentene
C) 2-methyl-2-pentene
D) 2,3-dimethyl-2-pentene
E) 2,4-dimethyl-2-pentene
A) 1-pentene
B) 2-pentene
C) 2-methyl-2-pentene
D) 2,3-dimethyl-2-pentene
E) 2,4-dimethyl-2-pentene

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
47
What effect does conjugation typically have on the frequency at which absorption by C  C occurs?
C occurs?
A) Conjugation decreases the frequency at which absorption occurs.
B) Conjugation increases the frequency at which absorption occurs.
C) Conjugation does not affect the frequency at which absorption occurs.
 C occurs?
C occurs?A) Conjugation decreases the frequency at which absorption occurs.
B) Conjugation increases the frequency at which absorption occurs.
C) Conjugation does not affect the frequency at which absorption occurs.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
48
What IR absorption is characteristic of the terminal C-H stretch in terminal alkynes?
A) 3500 cm-1
B) 3300 cm-1
C) 2400 cm-1
D) 2250 cm-1
E) 1710 cm-1
A) 3500 cm-1
B) 3300 cm-1
C) 2400 cm-1
D) 2250 cm-1
E) 1710 cm-1

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
49
How could you tell if the hydrogenation of 2-pentene to pentane was complete?

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
50
What m/z characterizes a strong peak in the mass spectrum of cyclopentanol?
A) 86
B) 85
C) 84
D) 70
E) 68
A) 86
B) 85
C) 84
D) 70
E) 68

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following most closely matches the C  C stretching frequency?
C stretching frequency?
A) 3300 cm-1
B) 2950 cm-1
C) 2200 cm-1
D) 1600 cm-1
E) 1200 cm-1
 C stretching frequency?
C stretching frequency?A) 3300 cm-1
B) 2950 cm-1
C) 2200 cm-1
D) 1600 cm-1
E) 1200 cm-1

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
52
Which compound would be expected to show intense IR absorption at 1746 cm-1?
A) CH3CH2OCH2CH3
B) CH3CH2CN
C) CH3CO2CH2CH3
D) CH3CH2SCH3
A) CH3CH2OCH2CH3
B) CH3CH2CN
C) CH3CO2CH2CH3
D) CH3CH2SCH3

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
53
Which compound lacks a strong, characteristic IR absorption near 1700 cm-1?
A) CH3CH2CO2H
B) CH3CH2CHO
C) (CH3CH2)2C=O
D) (CH3CH2)2O
E) H3CH2CO2CH3
A) CH3CH2CO2H
B) CH3CH2CHO
C) (CH3CH2)2C=O
D) (CH3CH2)2O
E) H3CH2CO2CH3

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
54
What IR absorption is characteristic of the C-H stretch in alkanes?
A) 3500 cm-1
B) 3300 cm-1
C) 2950 cm-1
D) 2250 cm-1
E) 1710 cm-1
A) 3500 cm-1
B) 3300 cm-1
C) 2950 cm-1
D) 2250 cm-1
E) 1710 cm-1

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
55
What IR absorption is characteristic of the O-H stretch in alcohols?
A) 4000 cm-1
B) 3300 cm-1
C) 2950 cm-1
D) 2250 cm-1
E) 1710 cm-1
A) 4000 cm-1
B) 3300 cm-1
C) 2950 cm-1
D) 2250 cm-1
E) 1710 cm-1

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
56
Which compound would be expected to show an IR absorption at 3300 cm-1?
A) CH3C CCH3
CCH3
B) butane
C) but-1-ene
D) CH3CH2C CH
CH
A) CH3C
 CCH3
CCH3B) butane
C) but-1-ene
D) CH3CH2C
 CH
CH
Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following compounds would contain characteristic IR stretches at 3300 and 2200 cm-1?
A) CH3CH2CHO
B) CH3CH=CHCH2OH
C) (CH3)2CHCN
D) CH3CH2CH2C≡CH
E) CH3C≡CCH2CH3
A) CH3CH2CHO
B) CH3CH=CHCH2OH
C) (CH3)2CHCN
D) CH3CH2CH2C≡CH
E) CH3C≡CCH2CH3

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following molecules would be expected to have its C=C stretching frequency at the highest wavenumber: benzene, 1,3-pentadiene, or 1-pentene?

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
59
Which compound would be expected to show intense IR absorption at 2820, 2710 and 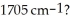
A) CH3COCH2CH3
B) PhCOCH3
C) PhCHO
D) CH2

CHCOCH3
A) CH3COCH2CH3
B) PhCOCH3
C) PhCHO
D) CH2

CHCOCH3

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
60
How could IR spectroscopy be used to distinguish between the following pair of compounds?
CH3CH2C
CH and CH3C
CCH3
CH3CH2C

CH and CH3C

CCH3

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following compounds has the lowest carbonyl stretching frequency? 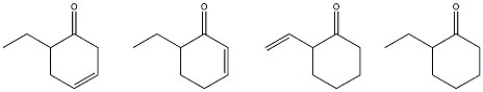


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
62
Explain the following observation regarding the IR stretching frequencies of the ketones shown below. 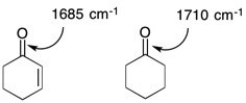


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following bonds, C-N, C 
N, or C
N, has an IR stretch around 1600 cm-1?

N, or C

N, has an IR stretch around 1600 cm-1?

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
64
Arrange the following IR bond stretches in order of increasing wavenumber:
C-O, C
C, O-H, and C=C.
C-O, C

C, O-H, and C=C.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
65
Which compound would be expected to show intense IR absorption at 2200 cm-1?
A) but-1-ene
B) PhCH2CN
C) CH3OCH2CH3
D) PhCH2NH2
A) but-1-ene
B) PhCH2CN
C) CH3OCH2CH3
D) PhCH2NH2

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
66
Which compound would be expected to show intense IR absorption at 1640 cm-1?
A) hex-1-ene
B) 2-methylheptane
C) CH3CH2CH2OH
D) CH3CH2COCH3
A) hex-1-ene
B) 2-methylheptane
C) CH3CH2CH2OH
D) CH3CH2COCH3

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
67
In addition to a carbonyl stretch, which of the following molecules exhibits two characteristic stretches at 2700 and 2800 cm-1?
A) (CH3CH2)2CO
B) CH3CH2CH2CHO
C) CH3CH2CO2CH3
D) CH3CH2CH2CO2H
E) CH3CH2CH2COCl
A) (CH3CH2)2CO
B) CH3CH2CH2CHO
C) CH3CH2CO2CH3
D) CH3CH2CH2CO2H
E) CH3CH2CH2COCl

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
68
Rank the following bonds in order of increasing stretching frequency (cm-1) in IR spectroscopy: 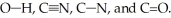
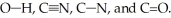

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
69
Which compound would you expect to see a broad absorption with two spikes around 3300 cm-1 and a strong absorption at 1640 cm-1?
A) CH2CH2CH2CONH2
B) CH2CH2CH2CONHCH3
C) CH2CH2CH2NH2
D) CH2CH2CH2NHCH3
A) CH2CH2CH2CONH2
B) CH2CH2CH2CONHCH3
C) CH2CH2CH2NH2
D) CH2CH2CH2NHCH3

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
70
Using IR, how could you tell if the reaction below was complete? 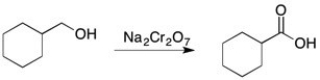


Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
71
How does the O-H stretch in the IR spectrum of a carboxylic acid differ from the O-H stretch of an alcohol?

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
72
How could IR spectroscopy be used to distinguish between the following pair of compounds?
(CH3)3N and CH3NHCH2CH3
(CH3)3N and CH3NHCH2CH3

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
73
What IR absorption is characteristic of the C=O stretch in aldehydes?
A) 3500 cm-1
B) 3300 cm-1
C) 2950 cm-1
D) 2250 cm-1
E) 1710 cm-1
A) 3500 cm-1
B) 3300 cm-1
C) 2950 cm-1
D) 2250 cm-1
E) 1710 cm-1

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following does not have a broad absorption with one or more spikes that is centered about 3300 cm-1 in the IR?
A) (CH3CH2CH2)3N
B) (CH3CH2CH2)2NH
C) CH3CH2CH2NH2
D) (CH3)3CNH2
E) (CH2=CHCH2)2NH
A) (CH3CH2CH2)3N
B) (CH3CH2CH2)2NH
C) CH3CH2CH2NH2
D) (CH3)3CNH2
E) (CH2=CHCH2)2NH

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
75
Which statement best explains why a carbonyl absorbs infrared radiation at a higher frequency than an alkene absorption?
A) Oxygen has a higher molecular mass than carbon.
B) A carbonyl bond is more polarized than an alkene bond.
C) Resonance delocalization of electrons invokes greater single bond character in a carbonyl bond.
D) A carbonyl π bond is much stronger than an alkene π bond.
A) Oxygen has a higher molecular mass than carbon.
B) A carbonyl bond is more polarized than an alkene bond.
C) Resonance delocalization of electrons invokes greater single bond character in a carbonyl bond.
D) A carbonyl π bond is much stronger than an alkene π bond.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
76
Rank the following bonds in order of increasing stretching frequency (cm-1) in IR spectroscopy: 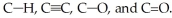
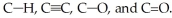

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
77
Using IR, how could you tell if the reaction below was complete? 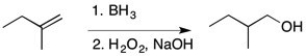
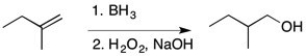

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
78
Deduce a possible structure for the compound with the IR absorptions below.
C3H5N: 2950, 2250 cm-1
C3H5N: 2950, 2250 cm-1

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
79
Which IR band is typically more intense, the C≡C stretch or the C≡N stretch? Explain briefly.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
80
Which compound would be expected to show intense IR absorption at 2250 cm-1?
A) CH3CH2CH2CO2H
B) (CH3)2CHCH2OH
C) (CH3)2CHCN
D) CH3CH2CH2CONH2
A) CH3CH2CH2CO2H
B) (CH3)2CHCH2OH
C) (CH3)2CHCN
D) CH3CH2CH2CONH2

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck


