Deck 1: Structure and Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/127
Play
Full screen (f)
Deck 1: Structure and Bonding
1
The formal charge on the nitrogen on the structure shown below is: 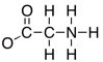

N = +1
2
Provide the electron configuration of phosphorus.
1s22s22p63s23p3
3
Draw the Lewis structure for 2-propanol, CH3CH(OH)CH3, including all non-bonding lone pairs.
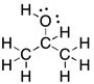
4
The electron density of ________ orbitals has spherical symmetry.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
5
How many distinct p orbitals exist in the second electron shell, where n = 2?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
6
A node is a region of high electron density between the two atoms in a covalent bond.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
7
Draw the shape of a 2p orbital, including shading to indicate phase.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
8
Which element in the second row of the periodic table has six valence electrons and a valence of two?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
9
Provide a Lewis structure for a molecule with molecular formula CH2O2.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
10
In a carbon atom, the 2s and 2p orbitals are the same energy.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
11
Atoms with the same number of protons but different numbers of neutrons are called ________.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
12
Draw the Lewis structure of acetic acid, CH3CO2H, including all non-bonding lone pairs.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
13
The ________ tells us that each orbital can hold a maximum of 2 electrons.
A) aufbau principle
B) Pauli exclusion principle
C) Hund's rule principle
D) LeChatelier principle
E) uncertainty principle
A) aufbau principle
B) Pauli exclusion principle
C) Hund's rule principle
D) LeChatelier principle
E) uncertainty principle

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
14
The atomic number of boron is 5. The correct electronic configuration of boron is:
A) 1s22s3
B) 1s22p3
C) 1s22s22p1
D) 2s22p3
E) 1s22s23s1
A) 1s22s3
B) 1s22p3
C) 1s22s22p1
D) 2s22p3
E) 1s22s23s1

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
15
While you were up late one night studying organic chemistry, you happened to see the last 5 minutes of an infomercial on TV. The spokesperson claimed that their brand of automobile tires were superior to all other brands on the market because they were made by using only natural rubber, isolated from the resin of rubber trees. How could a chemist test her claims that no petroleum products went into the manufacture of her brand of tires?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
16
An oxygen atom has ________ valence electrons.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
17
Orbitals which are equal in energy are referred to as ________.
A) degenerate
B) polar
C) nodes
D) filled
E) nonpolar
A) degenerate
B) polar
C) nodes
D) filled
E) nonpolar

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
18
When filling two or more orbitals of the same energy with electrons, the electrons will go into different orbitals rather than pair up in the same orbital.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
19
Draw a correct Lewis structure for chloromethane, CH3Cl, including all non-bonding lone pairs.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
20
The element with the electronic configuration 1s22s22p63s1 is ________.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
21
Add the appropriate formal charge to each atom in the molecule below. It is not necessary to indicate formal charges when zero. (All non-bonding electrons are included.) 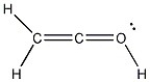
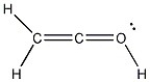

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
22
The formal charge on the oxygens in the compound below are ________. 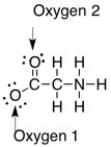
A) Oxygen 1: 0, Oxygen 2: 0
B) Oxygen 1: -1, Oxygen 2: 0
C) Oxygen 1: 0, Oxygen 2: -1
D) Oxygen 1: +1, Oxygen 2: 0
E) Oxygen 1: -1, Oxygen 2: -1

A) Oxygen 1: 0, Oxygen 2: 0
B) Oxygen 1: -1, Oxygen 2: 0
C) Oxygen 1: 0, Oxygen 2: -1
D) Oxygen 1: +1, Oxygen 2: 0
E) Oxygen 1: -1, Oxygen 2: -1

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
23
Add the appropriate formal charge to each atom in the molecule below. It is not necessary to indicate formal charges when zero. (All non-bonding electrons are included.) 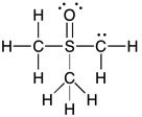


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
24
One or more of the atoms in the structure shown should have nonzero formal charges. Add the correct formal charge/s. (All non-bonding electrons have been included.) 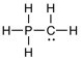


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
25
The formal charge on oxygen in dimethyl ether, CH3OCH3, is ________.
A) +2
B) +1
C) 0
D) -1
E) -2
A) +2
B) +1
C) 0
D) -1
E) -2

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
26
Draw a correct Lewis structure for acetonitrile, CH3CN, including all non-bonding lone pairs.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
27
Draw 2 possible Lewis structures for the compound with molecular formula C3H6.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
28
For most compounds in which a nitrogen atom bears no formal charge, the valence of this nitrogen atom is ________.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
29
Assign the correct formal charge to each nitrogen atom in the following Lewis structure. (All non-bonding electrons are included.) 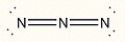
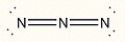

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
30
One or more of the atoms in the structure shown should have nonzero formal charges. Redraw the structure and the atoms with non-zero formal charges. 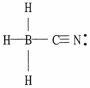


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
31
The electronegativity of elements on the periodic table increases going ________ a column and to the ________ in each row.
A) up; right
B) up; left
C) down; right
D) down; left
A) up; right
B) up; left
C) down; right
D) down; left

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following molecules contains a polar covalent bond?
A) H2
B) F2
C) CH3Cl
D) NaCl
E) He
A) H2
B) F2
C) CH3Cl
D) NaCl
E) He

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
33
A carbon-hydrogen bond in ethane (CH3CH3) is best described a ________.
A) highly polar
B) essentially nonpolar
C) ionic
D) a multiple bond
E) resonance stabilized
A) highly polar
B) essentially nonpolar
C) ionic
D) a multiple bond
E) resonance stabilized

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following are acceptable Lewis structures, including formal charges, for nitric acid, HNO3? 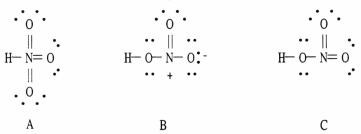
A) A only
B) B only
C) C only
D) both B and C
E) A, B, and C

A) A only
B) B only
C) C only
D) both B and C
E) A, B, and C

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
35
Draw a correct Lewis structure for tert-butyl alcohol, (CH3)3COH, including all non-bonding lone pairs.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
36
Covalent bonds may be polar or nonpolar. What property of the atoms forming a given bond determines this?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
37
Draw the Lewis structure for boric acid, B(OH)3, including all non-bonding lone pairs.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
38
Within a given row of the periodic table, electronegativity typically increases left to right across the row.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
39
The compound methylamine, CH3NH2, contains a C-N bond. In this bond, which of the following best describes the charge on the carbon atom?
A) +1
B) slightly positive
C) neutral
D) slightly negative
E) -1
A) +1
B) slightly positive
C) neutral
D) slightly negative
E) -1

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
40
Write a Lewis structure for a compound with the molecular formula H2N2.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
41
The Lewis structure of trimethylamine is shown below. Draw the condensed structural formula which corresponds to this Lewis structure. 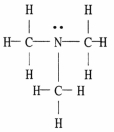


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
42
Nitroamines are common functional groups found in energetic materials, such as RDX and HMX. For the structure below, draw two other significant resonance structures, include any formal charges, and indicate the hybridization on each nitrogen and oxygen. 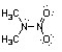
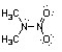

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following compounds are covalent compounds?
A) KCl
B) CF4
C) NH3
D) both A and B
E) both B and C
A) KCl
B) CF4
C) NH3
D) both A and B
E) both B and C

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
44
When a negatively charged species is most appropriately depicted as a hybrid of several resonance forms, the negative charge present is considered to be rapidly moving between the resonance forms bearing the formal negative charge.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
45
Structures ________, shown below, are resonance structures, and structure ________ is the major contributor to the overall resonance hybrid. 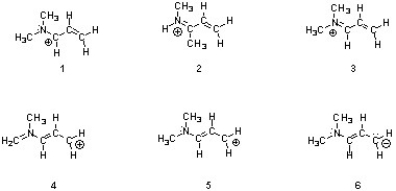
A) 2 & 4; 2
B) 1, 3 & 5; 3
C) 4 & 6; 6
D) 1, 3 & 5; 1
E) 1, 3, 4 & 5; 3

A) 2 & 4; 2
B) 1, 3 & 5; 3
C) 4 & 6; 6
D) 1, 3 & 5; 1
E) 1, 3, 4 & 5; 3

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
46
Draw the important resonance forms for the structure shown below. 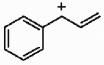


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
47
In the compound sodium methoxide (NaOCH3), there is ________ bonding.
A) ionic
B) polar covalent
C) nonpolar covalent
D) a mixture of ionic and covalent
E) resonance stabilized
A) ionic
B) polar covalent
C) nonpolar covalent
D) a mixture of ionic and covalent
E) resonance stabilized

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
48
Draw the complete Lewis structure for the compound whose condensed formula is (CH3)2CHCHO.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
49
One resonance structure of a cation is shown. Provide the other reasonable resonance structures. 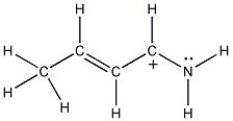


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
50
Draw the other important resonance form of: 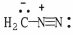
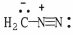

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
51
Draw a line-angle formula for (CH3)2CHCH2CH2NH2.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following structures (a-d) is another resonance structure of the following organic molecule? 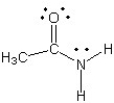
A)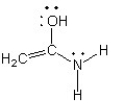
B)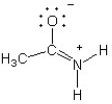
C)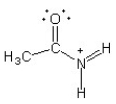
D)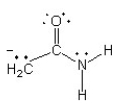

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
53
When a molecule can best be represented as a series of resonance forms, each of these forms always contributes to the same degree in the hybrid.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
54
The Lewis structure of pentane is shown below. Draw the condensed structural formula which corresponds to this Lewis structure. 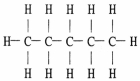
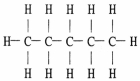

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
55
Draw 3 significant resonance structures for the compound shown below. Place a box around the major contributor. Fill in any missing formal charges. 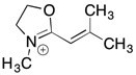


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
56
Draw additional resonance contributors for: 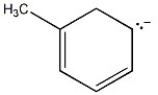


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following bonding patterns of carbon is not allowed in the formation of an organic compound?
A)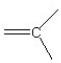
B)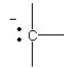
C)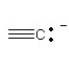
D)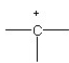
E)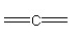
F)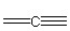
A)

B)

C)
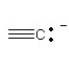
D)

E)
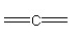
F)
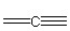

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
58
Draw the important resonance forms for the structure shown below. 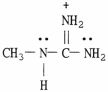


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following choices represent(s) a pair of resonance forms?
A)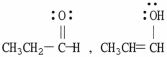
B)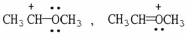
C)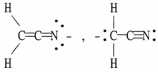
D) both A and C
E) both B and C
A)
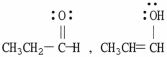
B)
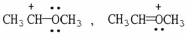
C)
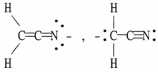
D) both A and C
E) both B and C

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
60
Draw the important resonance forms of the structure below to indicate the delocalization of charge. Indicate which is the major contributor to the overall structure. 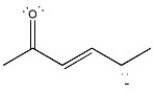


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
61
Draw the line-angle formula for three compounds with molecular formula C3H8O.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
62
What is the molecular formula for the following line-angle structure? 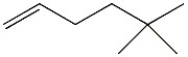


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following condensed formulas correctly represents the line-angle structure shown below? 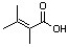
A) CH(CH3)2CH(CH3)CO2H
B) C2(CH3)3CO2H
C) (CH3)2CC(CH3)CO2H
D) C(CH3)2C(CH3)CH2CO2H

A) CH(CH3)2CH(CH3)CO2H
B) C2(CH3)3CO2H
C) (CH3)2CC(CH3)CO2H
D) C(CH3)2C(CH3)CH2CO2H

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
64
Provide the line-angle formula (skeletal structure) for (CH3)2CHCH2CHO.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following condensed formulas represents the same compound as the line-angle structure shown? 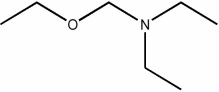
A) CH3CH2CH2OCH2CH2CH2N(CH2CH2CH3)2
B) CH3CH2CH2OCH2N(CH2CH3)2
C) CH3CH2OCH2N(CH2CH3)2
D) CH3CH2OCH2N(CH2CH2CH3)2
E) CH3ON(CH3)2

A) CH3CH2CH2OCH2CH2CH2N(CH2CH2CH3)2
B) CH3CH2CH2OCH2N(CH2CH3)2
C) CH3CH2OCH2N(CH2CH3)2
D) CH3CH2OCH2N(CH2CH2CH3)2
E) CH3ON(CH3)2

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
66
How many carbon atoms are present in the molecule shown? 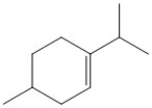
A) 6
B) 8
C) 10
D) 11
E) 12

A) 6
B) 8
C) 10
D) 11
E) 12

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
67
A condensed structure for acetone is CH3COCH3. Provide the structural formula for acetone.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
68
Draw a correct Lewis structure for CH3CHCHCOOH.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
69
Draw an acceptable line-angle formula for the compound shown below. 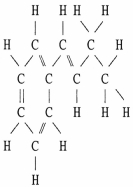


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
70
Provide the line-angle formula (skeletal structure) for (CH3CH2)2C=O.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
71
How many hydrogen atoms are present in the molecule shown? 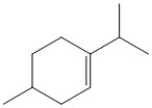


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
72
Draw a correct Lewis structure for acetaldehyde, CH3CHO.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
73
Compute the empirical and molecular formulas for the compound of molecular weight 180 g/mol which is shown to contain 40.0% C and 6.7% H by elemental analysis.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
74
Provide the line-angle formula for the alcohol CH3CH2CH(OH)CH2CH2CH(CH3)2.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
75
Provide the line-angle formula for CH3CH2C(CH3)2CH2CHO

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
76
Draw an acceptable line-angle formula for cyclobutanol (shown below). 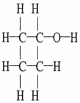


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
77
What is the molecular formula for the following line-angle structure? 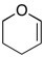


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
78
Draw a complete Lewis structure, including lone pairs, for (CH3)2CHCO2H.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
79
Draw condensed structures for the four compounds with formula C3H9N.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
80
Indicate the line-angle structure that corresponds to the condensed structure, HOCH2C(O)CH(CH3)2.
A)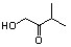
B)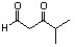
C)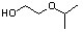
D)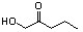
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck


