Deck 7: Substitution Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/103
Play
Full screen (f)
Deck 7: Substitution Reactions
1
When drawing an arrow-pushing mechanism,the tail of the arrow starts where?
A)At the bond that is being formed.
B)At the bond that is being broken.
C)At the source of electrons that is being moved.
D)At the location to which the electrons are being moved.
A)At the bond that is being formed.
B)At the bond that is being broken.
C)At the source of electrons that is being moved.
D)At the location to which the electrons are being moved.
At the bond that is being broken.
At the source of electrons that is being moved.
At the source of electrons that is being moved.
2
Provide an arrow pushing mechanism and product for the following reaction. 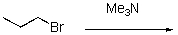
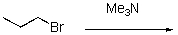
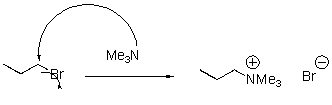
3
Provide an IUPAC name for the following compound. 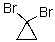
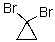
1,1-Dibromocyclopropane
4
Identify the labeled carbon as primary,secondary,or tertiary. 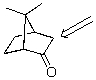


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
5
What are two features typical of a leaving group?

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
6
Provide examples of the 4 patterns for steps in the mechanisms of substitution reactions.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
7
Provide an IUPAC name for the following compound. 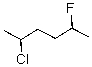


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is a reasonable definition of a concerted reaction?
A)It is a reaction which takes place in a series of steps.
B)It is a reaction which produces a loud noise.
C)It is a reaction in which all bond-breaking and bond-forming occurs at the same time.
D)It is a substitution reaction.
A)It is a reaction which takes place in a series of steps.
B)It is a reaction which produces a loud noise.
C)It is a reaction in which all bond-breaking and bond-forming occurs at the same time.
D)It is a substitution reaction.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
9
Draw an example of a substitution reaction.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is the correct IUPAC name of the following structure? 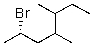
A)2-Bromo-3-butylpentane
B)(2S)-Bromo-4,5-dimethylheptane
C)3,4-Dimethyl-6-bromoheptane
D)2-Bromo-4-methylhexane

A)2-Bromo-3-butylpentane
B)(2S)-Bromo-4,5-dimethylheptane
C)3,4-Dimethyl-6-bromoheptane
D)2-Bromo-4-methylhexane

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
11
Provide an arrow pushing mechanism for the following reaction. 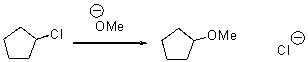


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
12
What is the leaving group in the following reaction? 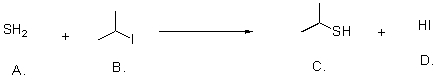
A)Sulfur
B)Carbon
C)Hydrogen
D)Iodide

A)Sulfur
B)Carbon
C)Hydrogen
D)Iodide

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
13
What is the leaving group in the following reaction? 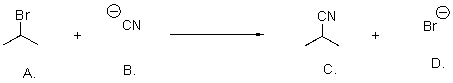
A)carbon
B)bromide
C)hydrogen
D)nitrogen

A)carbon
B)bromide
C)hydrogen
D)nitrogen

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
14
Identify the labeled carbon as primary,secondary,or tertiary. 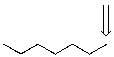


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
15
Identify the labeled carbon as primary,secondary,or tertiary. 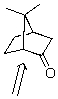


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
16
Provide a definition of a concerted reaction.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
17
For the following reaction,label the nucleophile,electrophile,and leaving group. 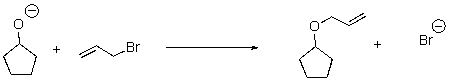


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is the correct IUPAC name of the following structure? 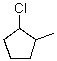
A)Chlorocyclopentane
B)2-Chloro-1-methylcyclopentane
C)1-Methyl-2-chlorocyclopentane
D)1-Chloro-2-methylcyclopentane

A)Chlorocyclopentane
B)2-Chloro-1-methylcyclopentane
C)1-Methyl-2-chlorocyclopentane
D)1-Chloro-2-methylcyclopentane

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is the correct IUPAC name of the following structure? 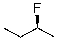
A)3-Fluorobutane
B)2-Fluorobutane
C)(S)-2-Fluorobutane
D)(R)-2-Fluorobutane

A)3-Fluorobutane
B)2-Fluorobutane
C)(S)-2-Fluorobutane
D)(R)-2-Fluorobutane

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
20
On the following compound,label all of the primary,secondary,and tertiary carbons. 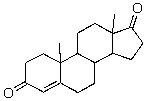


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
21
Rank the following substrates from most to least reactive in an SN2 reaction. 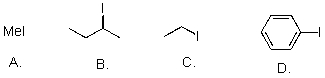
A)A>B>C>D
B)D>C>B>A
C)A>C>B>D
D).D>C>B>A

A)A>B>C>D
B)D>C>B>A
C)A>C>B>D
D).D>C>B>A

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
22
Rank the following substrates from most to least reactive in an SN2 reaction. 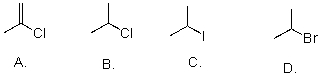
A)A>B>C>D
B)C>D>B>A
C)C>B>A>D
D)D>C>B>A

A)A>B>C>D
B)C>D>B>A
C)C>B>A>D
D)D>C>B>A

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
23
What is the product of the following reaction? 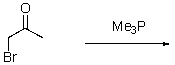
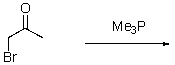

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
24
Provide an arrow-pushing mechanism for the following (intramolecular)reaction. 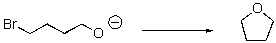
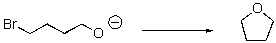

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
25
What is the product of the following reaction? 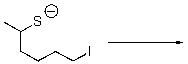
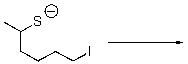

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
26
Provide the SN2 product of the following reaction. 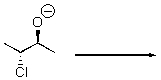
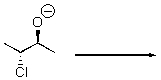

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
27
Rank the following substrates from most to least reactive in an SN2 reaction. 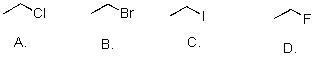
A)A>B>C>D
B)D>C>B>A
C)C>B>A>D
D)D>C>B>A.
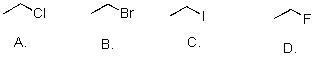
A)A>B>C>D
B)D>C>B>A
C)C>B>A>D
D)D>C>B>A.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
28
Given the following rate law,what will happen to the rate if the concentration of MeI is doubled? Rate = k[Me3N][MeI]
A)It will be doubled.
B)It will not change.
C)It will decrease in half.
D)It will increase very slightly.
A)It will be doubled.
B)It will not change.
C)It will decrease in half.
D)It will increase very slightly.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
29
Write the rate law for the following SN2 reaction. 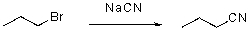
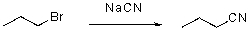

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
30
Draw a reaction diagram for the following reaction. 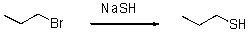
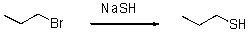

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is a mechanism for an SN2 reaction?
A)
B)
C)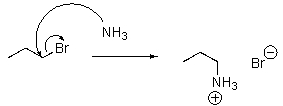
D)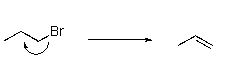
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
32
From the given starting material,how could you stereospecifically form the given product? 


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
33
Provide a mechanism for this reaction. 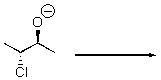
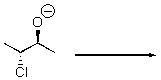

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
34
When drawing an arrow-pushing mechanism,the head of the arrow goes where?
A)At the bond that is being formed.
B)At the bond that is being broken.
C)At the source of electrons that is being moved.
D)At the location to which the electrons are being moved.
A)At the bond that is being formed.
B)At the bond that is being broken.
C)At the source of electrons that is being moved.
D)At the location to which the electrons are being moved.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is the rate law for the following SN2 reaction? ![<strong>Which of the following is the rate law for the following S<sub>N</sub>2 reaction? </strong> A)Rate = k[1-bromopropane] B)Rate = k[NaCN] C)Rate = k[NaCN][1-bromopropane] D)Rate = k[NaCN]<sup>2</sup>](https://storage.examlex.com/TB3186/11eab5f2_25a4_024b_a628_8fc70595f29b_TB3186_00.jpg)
A)Rate = k[1-bromopropane]
B)Rate = k[NaCN]
C)Rate = k[NaCN][1-bromopropane]
D)Rate = k[NaCN]2
![<strong>Which of the following is the rate law for the following S<sub>N</sub>2 reaction? </strong> A)Rate = k[1-bromopropane] B)Rate = k[NaCN] C)Rate = k[NaCN][1-bromopropane] D)Rate = k[NaCN]<sup>2</sup>](https://storage.examlex.com/TB3186/11eab5f2_25a4_024b_a628_8fc70595f29b_TB3186_00.jpg)
A)Rate = k[1-bromopropane]
B)Rate = k[NaCN]
C)Rate = k[NaCN][1-bromopropane]
D)Rate = k[NaCN]2

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
36
Rank the following substrates from most to least reactive in an SN2 reaction. 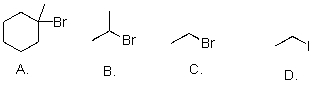
A)A>B>C>D
B)D>C>B>A
C)C>B>A>D
D)D>C>A>B

A)A>B>C>D
B)D>C>B>A
C)C>B>A>D
D)D>C>A>B

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
37
Provide the rate law for the following reaction. 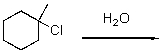
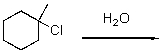

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is the rate law for the following reaction? ![<strong>Which of the following is the rate law for the following reaction? </strong> A)Rate = k[H<sub>2</sub>O] B)Rate = k[H<sub>2</sub>O][1-chloro-1-methylcyclohexane] C)Rate = k[1-chloro-1-methylcyclohexane] D)Rate = k[chloride ion]](https://storage.examlex.com/TB3186/11eab5f2_25a6_4c4f_a628_114f12a759af_TB3186_00.jpg)
A)Rate = k[H2O]
B)Rate = k[H2O][1-chloro-1-methylcyclohexane]
C)Rate = k[1-chloro-1-methylcyclohexane]
D)Rate = k[chloride ion]
![<strong>Which of the following is the rate law for the following reaction? </strong> A)Rate = k[H<sub>2</sub>O] B)Rate = k[H<sub>2</sub>O][1-chloro-1-methylcyclohexane] C)Rate = k[1-chloro-1-methylcyclohexane] D)Rate = k[chloride ion]](https://storage.examlex.com/TB3186/11eab5f2_25a6_4c4f_a628_114f12a759af_TB3186_00.jpg)
A)Rate = k[H2O]
B)Rate = k[H2O][1-chloro-1-methylcyclohexane]
C)Rate = k[1-chloro-1-methylcyclohexane]
D)Rate = k[chloride ion]

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
39
Given the following rate law,what will happen to the rate if the concentration of both reactants is doubled? Rate = k[Me3N][MeI]
A)It will be doubled.
B)It will not change.
C)It will decrease in half.
D)It will increase 4-fold.
A)It will be doubled.
B)It will not change.
C)It will decrease in half.
D)It will increase 4-fold.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
40
Given the following rate law,what will happen to the rate if the concentration of MeI is doubled and the concentration of Me3N is halved? Rate = k[Me3N][MeI]
A)It will be doubled.
B)It will not change.
C)It will decrease in half.
D)It will increase very slightly.
A)It will be doubled.
B)It will not change.
C)It will decrease in half.
D)It will increase very slightly.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following must be protonated in order to be a good leaving group?
A)Cl
B)Br
C)NMe2
D)OH2
A)Cl
B)Br
C)NMe2
D)OH2

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
42
What is the anticipated major product for the following reaction? 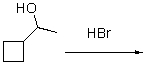


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
43
What is the mechanism for the following reaction? 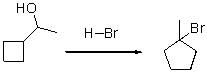


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
44
Draw a reaction diagram for the following SN1 reaction. 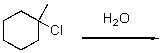

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
45
Circle the best leaving group in the following compound. 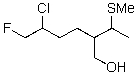


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
46
What is the best leaving group in the following compound? 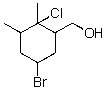


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
47
Provide an example of a nucleophile that will require a proton transfer step at the end of the reaction.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
48
Rank the following substrates from most to least reactive in an SN1 reaction. 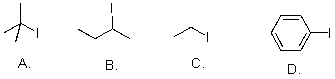
A)A>B>C>D
B)A>B>D>C
C)D>A>B>C
D)D>C>B>A

A)A>B>C>D
B)A>B>D>C
C)D>A>B>C
D)D>C>B>A

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
49
Why is a tertiary alkyl halide more reactive in an SN1 reaction than a secondary alkyl halide?

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is a good leaving group?
A)-OH
B)-CH3
C)-Br
D)-NH2
A)-OH
B)-CH3
C)-Br
D)-NH2

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
51
Draw the anticipated major product for the following reaction. 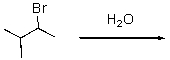
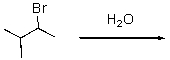

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
52
Rank the following substrates from most to least reactive in an SN1 reaction. 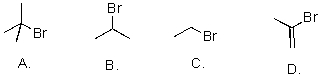
A)A>B>C>D
B)A>B>D>C
C)D>A>B>C
D)D>C>B>A

A)A>B>C>D
B)A>B>D>C
C)D>A>B>C
D)D>C>B>A

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
53
Draw a mechanism for the following reaction. 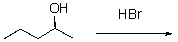
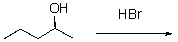

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following nucleophiles will require a proton transfer step at the end of the reaction?
A)NMe3
B)I-
C)OH-
D)H2S
A)NMe3
B)I-
C)OH-
D)H2S

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
55
Provide a mechanism for the following SN1 reaction. 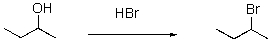


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
56
Rank the following substrates from most to least reactive in an SN1 reaction. 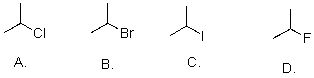
A)A>B>C>D
B)A>B>D>C
C)C>B>A>D
D)D>A>B>C

A)A>B>C>D
B)A>B>D>C
C)C>B>A>D
D)D>A>B>C

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
57
What is the product of the following reaction.Include stereochemistry. 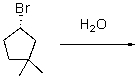


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
58
Why does a racemate form at the leaving group carbon in an SN1 reaction?

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
59
Provide the product of the following reaction. 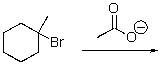


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
60
Rank the following substrates from most to least reactive in an SN1 reaction. 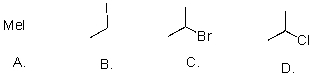
A)A>B>C>D
B)A>B>D>C
C)C>A>B>D
D)C>D>B>A

A)A>B>C>D
B)A>B>D>C
C)C>A>B>D
D)C>D>B>A

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
61
For the following reaction,provide an energy diagram. 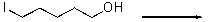
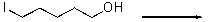

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
62
For the following reaction,which of the following is the mechanism? 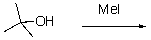
A)SN1
B)SN2
C)Protonation
D)Elimination
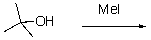
A)SN1
B)SN2
C)Protonation
D)Elimination

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
63
Is the following nucleophile strong or weak? I-
A)Strong
B)Weak
C)Not a nucleophile
A)Strong
B)Weak
C)Not a nucleophile

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
64
What set of reaction conditions should favor an SN1 reaction on 2-bromo-3-methylbutane?
A)weak nucleophile in a protic solvent
B)weak nucleophile in an aprotic solvent
C)strong nucleophile in a protic solvent
D)strong nucleophile in an aprotic solvent
A)weak nucleophile in a protic solvent
B)weak nucleophile in an aprotic solvent
C)strong nucleophile in a protic solvent
D)strong nucleophile in an aprotic solvent

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
65
What reaction mechanism is most likely for substitution on the following compound? 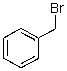
A)SN1
B)SN2
C)Either
D)Neither

A)SN1
B)SN2
C)Either
D)Neither

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
66
For the following reaction,provide the mechanism. 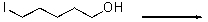
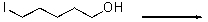

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
67
For the following reaction,which of the following is the mechanism? 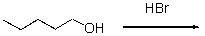
A)SN1
B)SN2
C)Protonation
D)Elimination
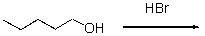
A)SN1
B)SN2
C)Protonation
D)Elimination

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
68
What reaction mechanism is most likely for substitution on the following compound? 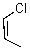
A)SN1
B)SN2
C)Either
D)Neither
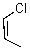
A)SN1
B)SN2
C)Either
D)Neither

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
69
What set of reaction conditions should favor an SN2 reaction on 2-bromo-3-methylbutane?
A)weak nucleophile in a protic solvent
B)weak nucleophile in an aprotic solvent
C)strong nucleophile in a protic solvent
D)strong nucleophile in an aprotic solvent
A)weak nucleophile in a protic solvent
B)weak nucleophile in an aprotic solvent
C)strong nucleophile in a protic solvent
D)strong nucleophile in an aprotic solvent

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
70
For the following reaction,draw the product. 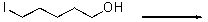
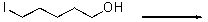

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
71
What reaction mechanism is most likely for substitution on the following compound? 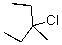
A)SN1
B)SN2
C)Either
D)Neither

A)SN1
B)SN2
C)Either
D)Neither

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
72
For the following reaction,provide the rate law. 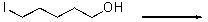
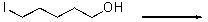

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
73
What reaction mechanism is most likely for substitution on the following compound? 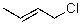
A)SN1
B)SN2
C)Either
D)Neither

A)SN1
B)SN2
C)Either
D)Neither

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
74
Draw an energy diagram for the following reaction. 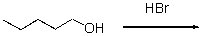
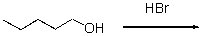

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
75
What reaction mechanism is most likely for substitution on the following compound? 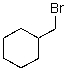
A)SN1
B)SN2
C)Either
D)Neither

A)SN1
B)SN2
C)Either
D)Neither

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
76
For the following reaction,which of the following is the rate law? ![<strong>For the following reaction,which of the following is the rate law? </strong> A)k[HBr] B)k[1-pentanol] C)k[HBr][1-pentanol] D)k[HBr]<sup>2</sup>](https://storage.examlex.com/TB3186/11eab5f2_25a9_80bd_a628_99fa2455a08c_TB3186_00.jpg)
A)k[HBr]
B)k[1-pentanol]
C)k[HBr][1-pentanol]
D)k[HBr]2
![<strong>For the following reaction,which of the following is the rate law? </strong> A)k[HBr] B)k[1-pentanol] C)k[HBr][1-pentanol] D)k[HBr]<sup>2</sup>](https://storage.examlex.com/TB3186/11eab5f2_25a9_80bd_a628_99fa2455a08c_TB3186_00.jpg)
A)k[HBr]
B)k[1-pentanol]
C)k[HBr][1-pentanol]
D)k[HBr]2

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
77
What is the rate law for the following SN1 reaction? 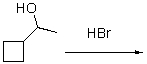


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
78
Draw an energy diagram for the following reaction. 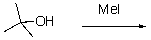
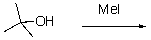

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
79
For the following reaction,which of the following is the rate law? ![<strong>For the following reaction,which of the following is the rate law? </strong> A)Rate = k[MeI] B)Rate = k[t-butanol] C)Rate = k[MeI][t-butanol] D)Rate = k[OH]](https://storage.examlex.com/TB3186/11eab5f2_25a9_f5f1_a628_85507e0d4d45_TB3186_00.jpg)
A)Rate = k[MeI]
B)Rate = k[t-butanol]
C)Rate = k[MeI][t-butanol]
D)Rate = k[OH]
![<strong>For the following reaction,which of the following is the rate law? </strong> A)Rate = k[MeI] B)Rate = k[t-butanol] C)Rate = k[MeI][t-butanol] D)Rate = k[OH]](https://storage.examlex.com/TB3186/11eab5f2_25a9_f5f1_a628_85507e0d4d45_TB3186_00.jpg)
A)Rate = k[MeI]
B)Rate = k[t-butanol]
C)Rate = k[MeI][t-butanol]
D)Rate = k[OH]

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
80
What reaction mechanism is most likely for substitution on the following compound? 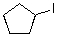
A)SN1
B)SN2
C)Either
D)Neither

A)SN1
B)SN2
C)Either
D)Neither

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck


