Deck 15: Infrared Spectroscopy and Mass Spectrometry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/108
Play
Full screen (f)
Deck 15: Infrared Spectroscopy and Mass Spectrometry
1
Rank the indicated bonds in decreasing (highest to lowest)order of wavenumber. 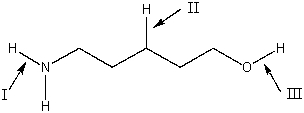
A)III>II>I
B)I>II>III
C)II>I>III
D)III>I>II
E)none of these

A)III>II>I
B)I>II>III
C)II>I>III
D)III>I>II
E)none of these
III>I>II
2
Which of the following information is primarily obtained from infrared spectroscopy?
A)arrangement of carbon and hydrogen atoms in a compound
B)molecular weight of a compound
C)conjugated system present in a compound
D)functional groups present in a compound
E)all of these
A)arrangement of carbon and hydrogen atoms in a compound
B)molecular weight of a compound
C)conjugated system present in a compound
D)functional groups present in a compound
E)all of these
functional groups present in a compound
3
Which one of the following compounds will have the lowest wavenumber for carbonyl absorption? 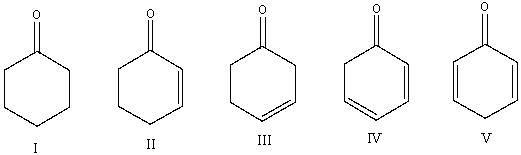
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V
IV
4
Which of the following compounds will have the highest wavenumber for carbonyl absorption? 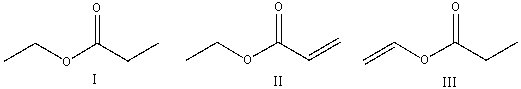
A)I
B)II
C)III
D)II & III

A)I
B)II
C)III
D)II & III

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
5
Arrange the following electromagnetic radiation in decreasing (highest to lowest)order of frequency. 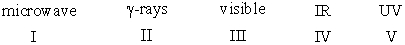
A)V>III>IV>II>I
B)II>V>III>IV>I
C)I>IV>III>V>II
D)V>II>IV>III>I
E)II>IV>V>III>I
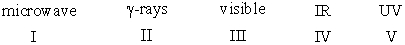
A)V>III>IV>II>I
B)II>V>III>IV>I
C)I>IV>III>V>II
D)V>II>IV>III>I
E)II>IV>V>III>I

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
6
Which one of the following compounds will have the lowest wavenumber for carbonyl absorption? 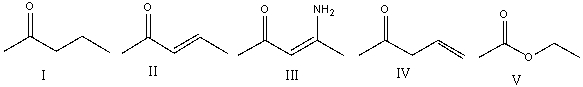
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
7
Absorption of _______radiation results in vibrational excitation of the bonds in a compound.
A)UV
B)Microwave
C)IR
D)Visible
E)x-ray
A)UV
B)Microwave
C)IR
D)Visible
E)x-ray

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following electromagnetic radiation has the highest frequency?
A)UV
B)X-ray
C)IR
D)microwave
E)visible
A)UV
B)X-ray
C)IR
D)microwave
E)visible

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following corresponds to the fingerprint region on an IR spectrum?
A)1500-4000cm-1
B)400-4000cm-1
C)400-1500cm-1
D)1500-2500cm-1
E)none of these
A)1500-4000cm-1
B)400-4000cm-1
C)400-1500cm-1
D)1500-2500cm-1
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
10
Which one of the following compounds will have the highest wavenumber for C=C absorption? 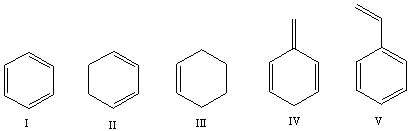
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following vibrations are observed in IR spectroscopy?
A)stetching
B)rotational
C)bending
D)A & B
E)A & C
A)stetching
B)rotational
C)bending
D)A & B
E)A & C

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is not true about electromagnetic radiation?
A)frequency is directly proportional to wavelength
B)frequency is directly proportional to energy
C)frequency is inversely proportional to wavelength
D)wavelength is inversely proportional to energy
E)none of these
A)frequency is directly proportional to wavelength
B)frequency is directly proportional to energy
C)frequency is inversely proportional to wavelength
D)wavelength is inversely proportional to energy
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following corresponds to the functional group region on an IR spectrum?
A)1500-4000cm-1
B)400-4000cm-1
C)400-1500cm-1
D)1500-2500cm-1
E)none of these
A)1500-4000cm-1
B)400-4000cm-1
C)400-1500cm-1
D)1500-2500cm-1
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following electromagnetic radiation has the highest energy?
A)UV
B)X-ray
C)IR
D)microwave
E)visible
A)UV
B)X-ray
C)IR
D)microwave
E)visible

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is currently used to indicate the location of a signal on an IR spectrum?
A)wavelength
B)wavenumber
C)frequency
D)A & B
E)all of these
A)wavelength
B)wavenumber
C)frequency
D)A & B
E)all of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following statement(s)is(are)true about the frequency of a stretching vibration according to Hook's law?
A)it is directly proportional to strength of the bond & the reduced mass
B)it is inversely proportional to strength of the bond & the reduced mass
C)it is directly proportional to strength of the bond
D)it is inversely proportional to the reduced mass
E)C & D
A)it is directly proportional to strength of the bond & the reduced mass
B)it is inversely proportional to strength of the bond & the reduced mass
C)it is directly proportional to strength of the bond
D)it is inversely proportional to the reduced mass
E)C & D

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
17
Rank the indicated bonds in decreasing (highest to lowest)order of wavenumber. 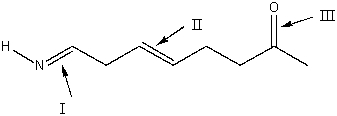
A)III>II>I
B)I>II>III
C)II>I>III
D)III>I>II
E)none of these

A)III>II>I
B)I>II>III
C)II>I>III
D)III>I>II
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
18
Rank the indicated bonds in decreasing (highest to lowest)order of wavenumber. 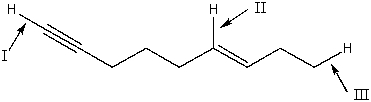
A)III>II>I
B)I>II>III
C)II>I>III
D)III>I>II
E)none of these

A)III>II>I
B)I>II>III
C)II>I>III
D)III>I>II
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following are units for wavenumber in IR spectroscopy?
A)cm-1
B)cm
C)J)s-1
D)mm
E)none of these
A)cm-1
B)cm
C)J)s-1
D)mm
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following electromagnetic radiation has the longest wavelength?
A)UV
B)X-ray
C)IR
D)microwave
E)visible
A)UV
B)X-ray
C)IR
D)microwave
E)visible

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
21
Arrange the indicated bonds in decreasing (highest to lowest)order of wavenumber. 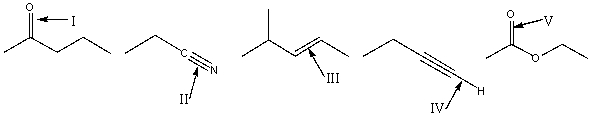
A)I>V>II>IV>III
B)IV>II>I>V>III
C)II>I>V>III>IV
D)IV>I>V>II>III
E)IV>II>V>I>III

A)I>V>II>IV>III
B)IV>II>I>V>III
C)II>I>V>III>IV
D)IV>I>V>II>III
E)IV>II>V>I>III

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following compounds will have the lowest wavenumber for carbonyl absorption? 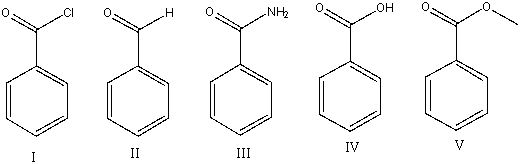
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following compounds will show a sharp absorption at 3300 cm-1 and at 2150 cm-1? 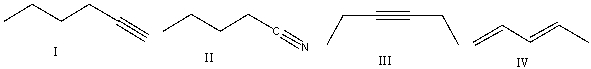
A)I
B)II
C)III
D)IV
E)none of these
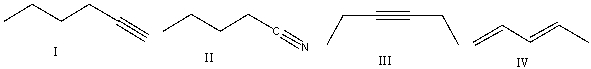
A)I
B)II
C)III
D)IV
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following bonds would produce the strongest absorption?
A)C=N
B)C C
C)C=O
D)sp2C-H
E)C-O
A)C=N
B)C C
C)C=O
D)sp2C-H
E)C-O

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following bonds is IR inactive? 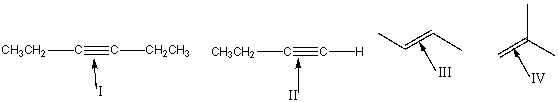
A)I
B)II
C)III
D)I & IV
E)I & III

A)I
B)II
C)III
D)I & IV
E)I & III

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
26
How can you distinguish between cyclohexanol and cyclohexanecarboxylic acid using IR spectroscopy? 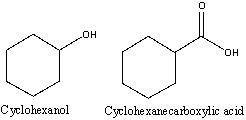


Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following compounds will show absorption at 2250 cm-1? 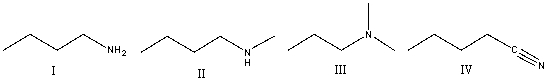
A)I
B)II
C)III
D)IV
E)none of these

A)I
B)II
C)III
D)IV
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following bonds would produce the weakest absorption?
A)C=C
B)O-H
C)C=O
D)sp3C-H
E)A & D
A)C=C
B)O-H
C)C=O
D)sp3C-H
E)A & D

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
29
Diluted alcohols show a _____absorption around 3600cm-1,due to _____.
A)sharp,hydrogen bonding
B)broad,hydrogen bonding
C)sharp,absence of hydrogen bonding
D)broad,absence of hydrogen bonding
A)sharp,hydrogen bonding
B)broad,hydrogen bonding
C)sharp,absence of hydrogen bonding
D)broad,absence of hydrogen bonding

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following alkene groups will produce the strongest signal? 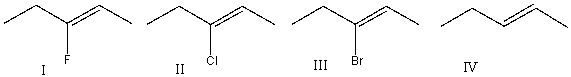
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
31
A compound with molecular formula C6H10O,shows absorptions at 1720 cm-1 and at 2980 cm-1 on the IR spectrum.Propose a possible structure for this compound.

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
32
Concentrated alcohols show a _____absorption in the region of 3200-3600cm-1,due to _____.
A)sharp,hydrogen bonding
B)broad,hydrogen bonding
C)sharp,polarity
D)broad,polarity
A)sharp,hydrogen bonding
B)broad,hydrogen bonding
C)sharp,polarity
D)broad,polarity

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following compounds will show a broad absorption around 3300 cm-1 and at 1650 cm-1? 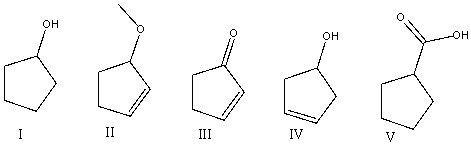
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
34
Primary amines show two medium absorption bands around 3400 cm-1,due to _______.
A)symmetric stretching
B)asymmetric stretching
C)both symmetric and asymmetric stretching
D)hydrogen bonding
A)symmetric stretching
B)asymmetric stretching
C)both symmetric and asymmetric stretching
D)hydrogen bonding

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following statement(s)is(are)true for an IR-active bond?
A)the bond must be symmetrical
B)it must change bond length
C)it must change bond angle
D)it must change bond dipole
E)all of these
A)the bond must be symmetrical
B)it must change bond length
C)it must change bond angle
D)it must change bond dipole
E)all of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
36
A compound with molecular formula C3H9N,shows absorptions at 3400 cm-1 (two),2980 cm-1 and at 1100 cm-1 on the IR spectrum.Propose a possible structure for this compound.

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following compounds will show absorptions at 1640 cm-1,2950 cm-1 and 3050 cm-1 on the IR spectrum? 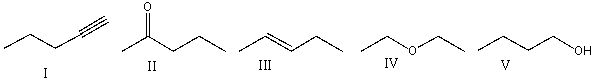
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is true about the IR spectrum of the following compound? 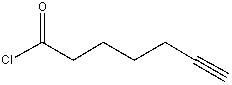
A)absorptions at 1720 cm-1 & 2150 cm-1
B)absorptions at 1800 cm-1 & 2150 cm-1
C)absorptions at 1720 cm-1 & 2250 cm-1
D)absorptions at 1800 cm-1 & 2250 cm-1
E)absorptions at 1650 cm-1 & 2150 cm-1

A)absorptions at 1720 cm-1 & 2150 cm-1
B)absorptions at 1800 cm-1 & 2150 cm-1
C)absorptions at 1720 cm-1 & 2250 cm-1
D)absorptions at 1800 cm-1 & 2250 cm-1
E)absorptions at 1650 cm-1 & 2150 cm-1

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following compounds will show two absorptions at 2600 cm-1 and at 2700 cm-1 along with the carbonyl absorption? 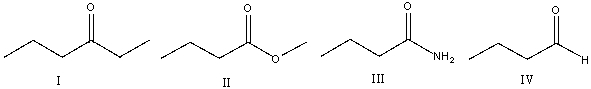
A)I
B)II
C)III
D)IV
E)none of these

A)I
B)II
C)III
D)IV
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
40
Carboxylic acids show a very broad absorption for the OH compared to alcohols,because they can form a ______.
A)dimer
B)polymer
C)trimer
D)tetramer
A)dimer
B)polymer
C)trimer
D)tetramer

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is true about CH3CH3+ . ?
A)it is the parent ion of ethane
B)it is a molecular ion of ethane with m/z =30
C)it is a fragment of propane
D)A & B
E)none of these
A)it is the parent ion of ethane
B)it is a molecular ion of ethane with m/z =30
C)it is a fragment of propane
D)A & B
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
42
Which one of the following compounds is consistent with the following IR spectrum? 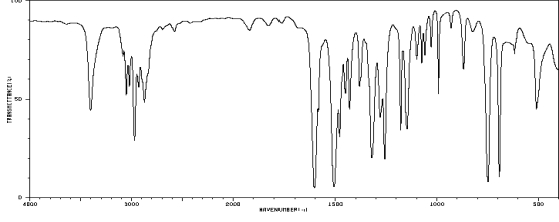 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 
A)I
B)II
C)III
D)IV
E)V
 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
43
Mass spectrometry is primarily used to determine:
A)molecular formula of a compound
B)molecular weight of a compound
C)conjugation in a compound
D)A & B
E)A & C
A)molecular formula of a compound
B)molecular weight of a compound
C)conjugation in a compound
D)A & B
E)A & C

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following compounds will have two absorptions at 1820 cm-1 and 1740cm-1? 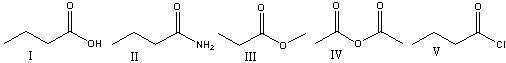
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is not true about the molecular ion in mass spectrometry?
A)The molecular ion is produced by loss of one electron from the molecule.
B)The mass of the molecular ion is equivalent to the mass of the molecule.
C)The ion is produced by a loss of pair of electrons from the molecule.
D)The molecular ion is often unstable and can undergo fragmentation.
E)none of these
A)The molecular ion is produced by loss of one electron from the molecule.
B)The mass of the molecular ion is equivalent to the mass of the molecule.
C)The ion is produced by a loss of pair of electrons from the molecule.
D)The molecular ion is often unstable and can undergo fragmentation.
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following compounds will have highest wavenumber for carbonyl absorption? 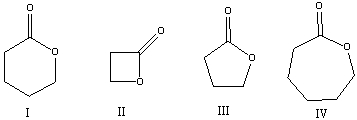
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
47
Predict the product for the following reaction and explain how you will use IR spectroscopy to monitor the progress of the reaction. 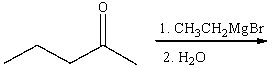
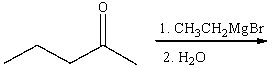

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
48
For the following reaction,which of the following is consistent with the IR spectrum of the product? 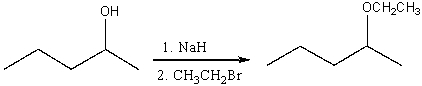
A)absorption at 3200-3600 cm-1 should disappear
B)absorption at 3200-3600 cm-1 and 1100 cm-1 should disappear
C)absorption at 1100 cm-1 should disappear and absorption at 3100 cm-1 should appear
D)absorption at 1650 cm-1 should disappear and absorption at 3300 cm-1 should appear.
E)none of these
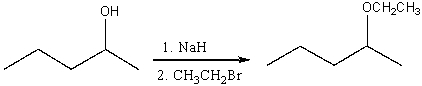
A)absorption at 3200-3600 cm-1 should disappear
B)absorption at 3200-3600 cm-1 and 1100 cm-1 should disappear
C)absorption at 1100 cm-1 should disappear and absorption at 3100 cm-1 should appear
D)absorption at 1650 cm-1 should disappear and absorption at 3300 cm-1 should appear.
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is always true about the base peak in a mass spectrum?
A)peak corresponding to molecular ion
B)peak corresponding to the most abundant ion
C)peak corresponding to lowest m/z
D)A & C
E)none of these
A)peak corresponding to molecular ion
B)peak corresponding to the most abundant ion
C)peak corresponding to lowest m/z
D)A & C
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
50
For the following reaction,which of the following is consistent with the IR spectrum of the product? 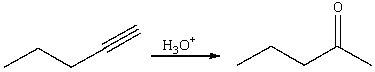
A)absorption at 2150 cm-1 should disappear
B)absorption at 3300 cm-1 and 2150cm-1 should disappear
C)absorption at 2250 cm-1 should disappear,new absorption at 3300 cm-1 should appear
D)absorption at 1650 cm-1 should disappear and absorption at 3300 cm-1 should appear
E)absorption at 3300 cm-1 and 2150cm-1 should disappear,a new absorption at 1720 cm-1 should appear

A)absorption at 2150 cm-1 should disappear
B)absorption at 3300 cm-1 and 2150cm-1 should disappear
C)absorption at 2250 cm-1 should disappear,new absorption at 3300 cm-1 should appear
D)absorption at 1650 cm-1 should disappear and absorption at 3300 cm-1 should appear
E)absorption at 3300 cm-1 and 2150cm-1 should disappear,a new absorption at 1720 cm-1 should appear

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
51
The separation of ions in the mass spectrometer is done by their ______.
A)electrons to protons ratio
B)mass to neutrons ratio
C)protons to neutrons ratio
D)mass to charge ratio
E)none of these
A)electrons to protons ratio
B)mass to neutrons ratio
C)protons to neutrons ratio
D)mass to charge ratio
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
52
Which one of the following compounds is consistent with the following IR spectrum? 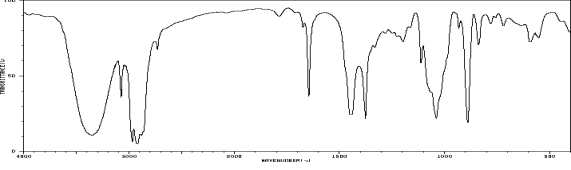 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 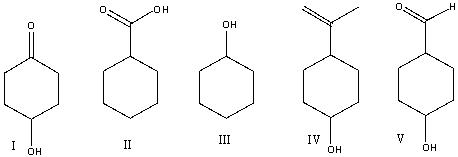
A)I
B)II
C)III
D)IV
E)V
 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
53
Which one of the following compounds is consistent with the following IR spectrum? 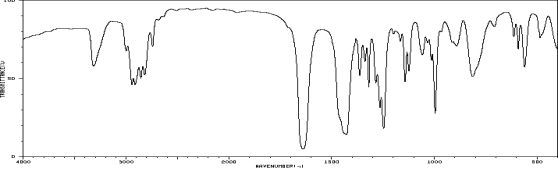 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 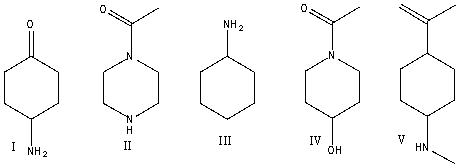
A)I
B)II
C)III
D)IV
E)V
 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
54
For the following reaction,which of the following is consistent with the IR spectrum of the product? 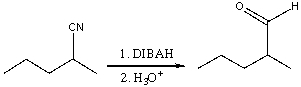
A)absorption at 2250 cm-1 should disappear
B)absorption at 3200-3400 cm-1 and 1720cm-1 should appear
C)absorption at 2250 cm-1 should disappear,new absorption at 2600-2800 cm-1 and 1720 cm-1 should appear.
D)absorption at 2250 cm-1 should disappear and absorption around 3400 cm-1 should appear.
E)none of these
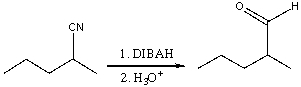
A)absorption at 2250 cm-1 should disappear
B)absorption at 3200-3400 cm-1 and 1720cm-1 should appear
C)absorption at 2250 cm-1 should disappear,new absorption at 2600-2800 cm-1 and 1720 cm-1 should appear.
D)absorption at 2250 cm-1 should disappear and absorption around 3400 cm-1 should appear.
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
55
A compound with molecular formula C4H8O2,shows absorptions at 2200-3600 cm-1 (broad),1720 cm-1 and at 1200 cm-1 on the IR spectrum.Propose a possible structure for this compound.

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
56
Which one of the following compounds is consistent with the following IR spectrum? 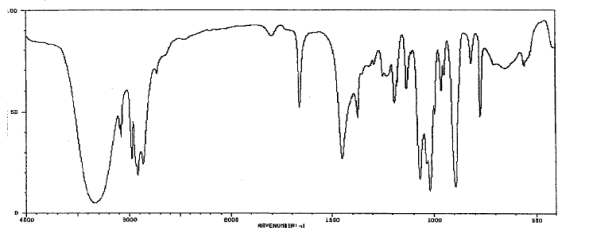 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 
A)I
B)II
C)III
D)IV
E)V
 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
57
Which one of the following compounds is consistent with the following IR spectrum? 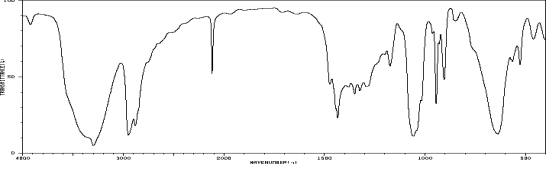 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 
A)I
B)II
C)III
D)IV
E)V
 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is initially produced when a compound is bombarded with high energy electrons?
A)anion
B)free radical
C)radical cation
D)cation
E)none of these
A)anion
B)free radical
C)radical cation
D)cation
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
59
Which one of the following compounds is consistent with the following IR spectrum? 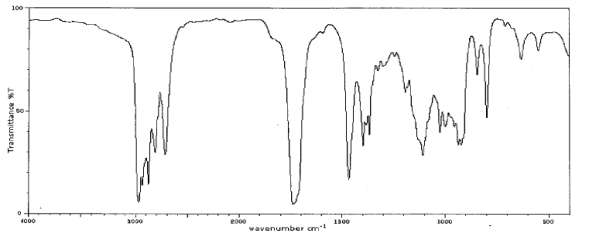 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 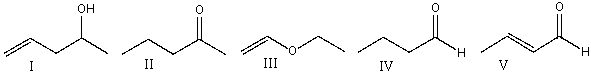
A)I
B)II
C)III
D)IV
E)V
 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
60
Predict the product for the following reaction and explain how you will use IR spectroscopy to monitor the progress of the reaction. 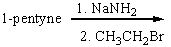
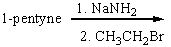

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
61
Provide a molecular formula that is consistent with the following mass spectrum data.M+ . at m/z= 136,relative height=65.6%
(M+1)+ . at m/z= 137,relative height=6.2%
(M+1)+ . at m/z= 137,relative height=6.2%

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the m/z values correspond to the molecular ion for the following compound? 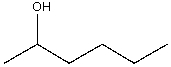
A)18
B)82
C)100
D)102
E)103

A)18
B)82
C)100
D)102
E)103

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
63
For which of the following compounds will the (M+2)+ . peak intensity be around 25% of the intensity of the molecular ion peak?
A)CH3CH2CH2Br
B)CH3CH2CH2OH
C)CH3CH2CH2Cl
D)CH3CH2CH2NH2
E)none of these
A)CH3CH2CH2Br
B)CH3CH2CH2OH
C)CH3CH2CH2Cl
D)CH3CH2CH2NH2
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
64
Compounds containing chlorine or bromine usually show a strong _____peak.
A)molecular ion
B)base
C)M+1
D)M+2
E)all of these
A)molecular ion
B)base
C)M+1
D)M+2
E)all of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the m/z values correspond to the molecular ion peak in the following mass spectrum? 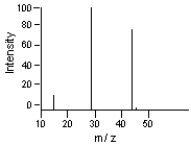
A)45
B)44
C)29
D)15
E)none of these

A)45
B)44
C)29
D)15
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following will produce M+ . and (M+2)+ . peaks of equal intensity?
A)nitrogen
B)chlorine
C)bromine
D)oxygen
E)B & C
A)nitrogen
B)chlorine
C)bromine
D)oxygen
E)B & C

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following is true about the (M+1)+ . peak on the mass spectrum of a hydrocarbon?
A)it is the most abundant peak
B)it is due to the 13C isotope of carbon
C)it has a m/z value lower than the molecular ion
D)it is useful in calculating number of carbon atoms
E)B & D
A)it is the most abundant peak
B)it is due to the 13C isotope of carbon
C)it has a m/z value lower than the molecular ion
D)it is useful in calculating number of carbon atoms
E)B & D

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
68
Provide a molecular formula that is consistent with the following mass spectrum data.M+ . at m/z= 167,relative height=50.0%
(M+1)+ . at m/z= 168,relative height=4.4%
(M+1)+ . at m/z= 168,relative height=4.4%

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following compounds will have odd m/z value for the molecular ion? 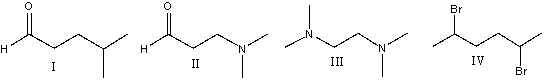
A)I
B)II
C)III
D)IV
E)none of these

A)I
B)II
C)III
D)IV
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
70
Which molecular formula is consistent with the following mass spectrum data? M+ . at m/z= 108,relative height=61.5%
(M+1)+ . at m/z= 109,relative height=1.5%
(M+2)+ . at m/z= 110,relative height=61.3%
A)C2H5Br
B)C5H12Cl
C)C8H12
D)C2H7Br
E)none of these
(M+1)+ . at m/z= 109,relative height=1.5%
(M+2)+ . at m/z= 110,relative height=61.3%
A)C2H5Br
B)C5H12Cl
C)C8H12
D)C2H7Br
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
71
Provide a molecular formula that is consistent with the following mass spectrum data.M+ . at m/z= 73,relative height=86.1%
(M+1)+ . at m/z= 74,relative height=3.2%
(M+1)+ . at m/z= 74,relative height=3.2%

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following is true about the molecular weight and the M+ . -m/z value for the following compound? 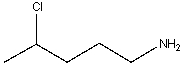
A)odd molecular weight,m/z-115
B)odd molecular weight,m/z-121
C)even molecular weight,m/z-96
D)even molecular weight,m/z-132
E)none of these

A)odd molecular weight,m/z-115
B)odd molecular weight,m/z-121
C)even molecular weight,m/z-96
D)even molecular weight,m/z-132
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following compounds will produce a prominent (M-18)peak in the mass spectrum?
A)2-methylheptane
B)1-heptanol
C)heptanamine
D)heptanal
E)none of these
A)2-methylheptane
B)1-heptanol
C)heptanamine
D)heptanal
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following mass spectra shows the presence of bromine in a compound? 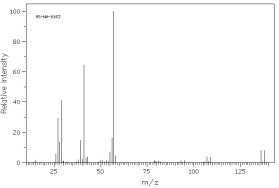
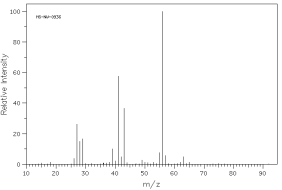 I II
I II 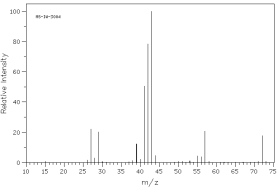
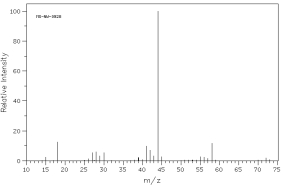 III IV Four spectra above: courtesy of SDBS: National Institute of Advanced Industrial Science and Technology
III IV Four spectra above: courtesy of SDBS: National Institute of Advanced Industrial Science and Technology
A)I
B)II
C)III
D)IV
E)none of these

 I II
I II 
 III IV Four spectra above: courtesy of SDBS: National Institute of Advanced Industrial Science and Technology
III IV Four spectra above: courtesy of SDBS: National Institute of Advanced Industrial Science and TechnologyA)I
B)II
C)III
D)IV
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
75
Presence of bromine produces M+ . and (M+2)+ . peaks of equal intensity on a mass spectrum because:
A)81Br isotope has higher natural abundance than 79Br isotope
B)79Br and 81Br isotopes have almost equal natural abundance
C)79Br isotope has higher natural abundance than 81Br isotope
D)none of these
A)81Br isotope has higher natural abundance than 79Br isotope
B)79Br and 81Br isotopes have almost equal natural abundance
C)79Br isotope has higher natural abundance than 81Br isotope
D)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the m/z values corresponds to the base peak in the following mass spectrum? 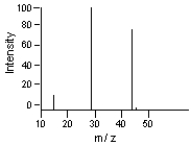
A)45
B)44
C)29
D)15
E)none of these

A)45
B)44
C)29
D)15
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following compounds will produce a prominent (M-15)peak in the mass spectrum?
A)2-methylheptane
B)1-heptanol
C)heptanamine
D)1-chloroheptane
E)none of these
A)2-methylheptane
B)1-heptanol
C)heptanamine
D)1-chloroheptane
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
78
Which molecular formula is consistent with the following mass spectrum data? M+ . at m/z= 78,relative height=23.5%
(M+1)+ . at m/z= 79,relative height=0.78%
(M+2)+ . at m/z= 80,relative height=7.5%
A)C6H6
B)C3H7Cl
C)C6H8
D)C6H9Cl
E)none of these
(M+1)+ . at m/z= 79,relative height=0.78%
(M+2)+ . at m/z= 80,relative height=7.5%
A)C6H6
B)C3H7Cl
C)C6H8
D)C6H9Cl
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
79
Which molecular formula is consistent with the following mass spectrum data? M+ . at m/z= 72,relative height=73.0%
(M+1)+ . at m/z= 73,relative height=3.3%
A)C4H10O
B)C4H9N
C)C5H12
D)C4H8O
E)none of these
(M+1)+ . at m/z= 73,relative height=3.3%
A)C4H10O
B)C4H9N
C)C5H12
D)C4H8O
E)none of these

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
80
Which molecular formula is consistent with the following mass spectrum data? M+ . at m/z= 84,relative height=10.0%
(M+1)+ . at m/z= 85,relative height=0.56%
A)C5H10O
B)C5H8O
C)C5H24
D)C6H12
E)C4H6O2
(M+1)+ . at m/z= 85,relative height=0.56%
A)C5H10O
B)C5H8O
C)C5H24
D)C6H12
E)C4H6O2

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck


