Deck 6: Properties and Reactions of Haloalkanes: Bimolecular Nucleophilic Substitution
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/38
Play
Full screen (f)
Deck 6: Properties and Reactions of Haloalkanes: Bimolecular Nucleophilic Substitution
1
What would be the organic product of the following reaction? 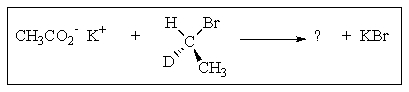
A)
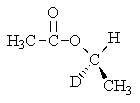
B)
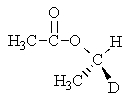
C)
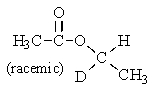
D) All of the above.
E) None of the above.

A)

B)

C)
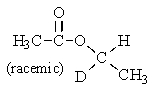
D) All of the above.
E) None of the above.

2
What is the major product of the following reaction: 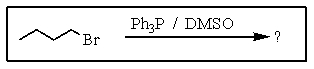
A)
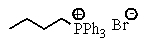
B)
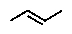
C)
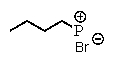
D)
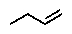
E) no reaction occurs

A)

B)

C)

D)

E) no reaction occurs

3
What is the major product of the following two-step reaction? 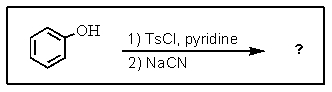
A)
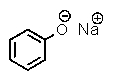
B)
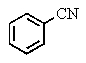
C)
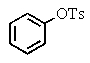
D)
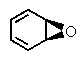
E) no reaction occurs

A)

B)

C)

D)

E) no reaction occurs

4
To which side (if any)would the following equilibrium lie? 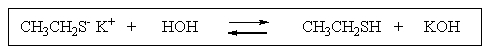
A) to the left
B) to the right
C) equally to the right and left
D) there is no way to tell
E) only SN2,SN1 and E2 reactions are possible

A) to the left
B) to the right
C) equally to the right and left
D) there is no way to tell
E) only SN2,SN1 and E2 reactions are possible

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following reagents would best accomplish a typical SN2 reaction?
A) CH3OH
B) H2O
C) HCN
D) KCN
E) KOtBu
A) CH3OH
B) H2O
C) HCN
D) KCN
E) KOtBu

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following haloalkanes would not undergo the reaction below? 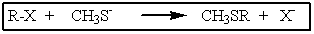
A) (CH3)2CHI
B) CH3Cl
C) (CH3)3CBr
D) CH3CH2Br
E) CH3CH2CH2I

A) (CH3)2CHI
B) CH3Cl
C) (CH3)3CBr
D) CH3CH2Br
E) CH3CH2CH2I

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
7
What reactants are required to achieve the following transformation? 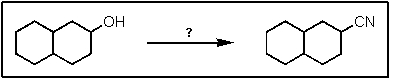
A)
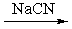
B)
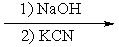
C)
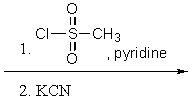
D)
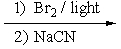
E) None of the above.

A)
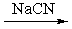
B)
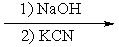
C)

D)
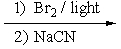
E) None of the above.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
8
What would be the major product of the following reaction? 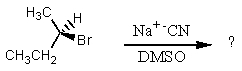
A)
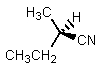
B)
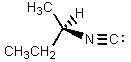
C)
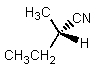
D)
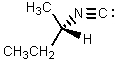
E)
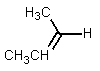
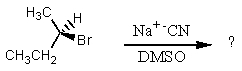
A)

B)

C)

D)
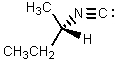
E)


Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
9
What would be the name of the following? 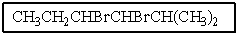
A) 3,4-dibromo-2-methylhexane
B) 1,2-dibromo-1-isopropylbutane
C) 2,3-dibromohexane
D) 3,4-dibromo-5-methylhexane
E) 3,4-dibromo-3-methylhexane

A) 3,4-dibromo-2-methylhexane
B) 1,2-dibromo-1-isopropylbutane
C) 2,3-dibromohexane
D) 3,4-dibromo-5-methylhexane
E) 3,4-dibromo-3-methylhexane

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following would you expect to react fastest with the nucleophile I- (iodide)?
A) CH3CH2CH2Br
B) CH3CH2CH2Cl
C) (CH3)2CHCH2Br
D) (CH3)2CHCH2Cl
E) (CH3)3CCH2Br
A) CH3CH2CH2Br
B) CH3CH2CH2Cl
C) (CH3)2CHCH2Br
D) (CH3)2CHCH2Cl
E) (CH3)3CCH2Br

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following would you expect to have the weakest C-X bond?
A) CH3Cl
B) CH3CH2Br
C) CH3F
D) CH3CH2I
E) (CH3)2CHBr
A) CH3Cl
B) CH3CH2Br
C) CH3F
D) CH3CH2I
E) (CH3)2CHBr

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
12
Indicate the reagents required to achieve the following transformation: 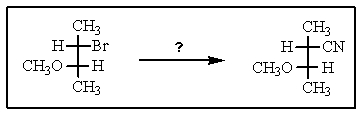
A)
B)
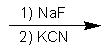
C)
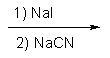
D)
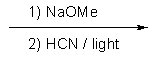
E) None of the above.

A)
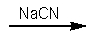
B)
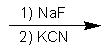
C)
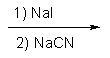
D)
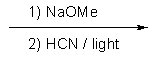
E) None of the above.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
13
Complete the following reaction: 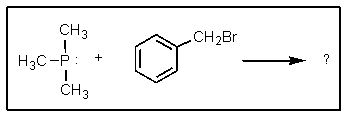
A)
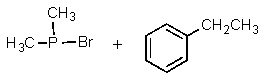
B)
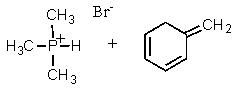
C)
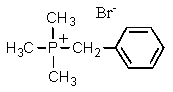
D)
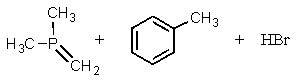
E) no reaction will occur

A)

B)

C)

D)

E) no reaction will occur

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is not normally a good leaving group on carbon?
A) Br
B) OCH3
C) Cl
D) OSO2R
E) I
A) Br
B) OCH3
C) Cl
D) OSO2R
E) I

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
15
Predict the major product of the following reaction: 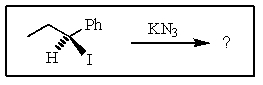
A)
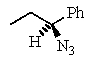
B)
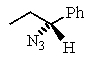
C)
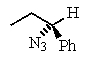
D)
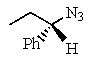
E) no reaction occurs

A)

B)

C)
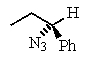
D)

E) no reaction occurs

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
16
Several alkyl halides,including iodomethane,are known carcinogens or cancer-suspect materials.To destroy these materials by conversion to non-electrophilic species,you can react them with nucleophiles.Which of the following would be the best for rapidly destroying methyl iodide (iodomethane)?
A) CH3OH
B) NH3
C) H2O
D) NaI
E) CH3CO2H
A) CH3OH
B) NH3
C) H2O
D) NaI
E) CH3CO2H

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
17
What reactants are required to achieve the following transformation? 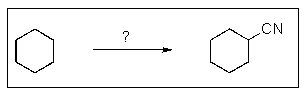
A)
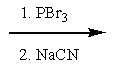
B)
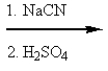
C)
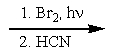
D)
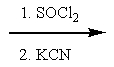
E)
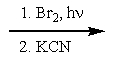

A)

B)

C)
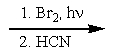
D)

E)


Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following would you expect to have the highest boiling point?
A) CH3CH2CH2Br
B) CH3CH2CH2I
C) CH3CH2CH2Cl
D) CH3CH2CH2F
E) CH3CH2CH3
A) CH3CH2CH2Br
B) CH3CH2CH2I
C) CH3CH2CH2Cl
D) CH3CH2CH2F
E) CH3CH2CH3

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
19
Predict the major product of the following reaction: 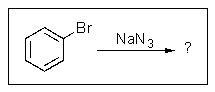
A)
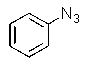
B)
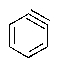
C)
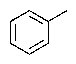
D)
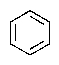
E) no reaction occurs

A)

B)

C)

D)

E) no reaction occurs

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
20
Predict the major product of the following reaction: 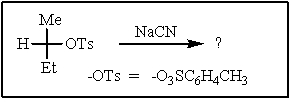
A)
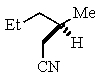
B)
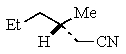
C)
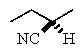
D)
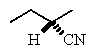
E)
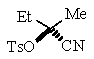

A)

B)
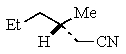
C)

D)

E)
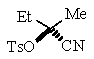

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
21
Arrange the following in order of increasing nucleophilicity. 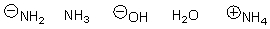
A)
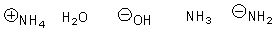
B)
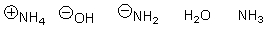
C)
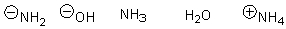
D)
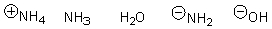
E)
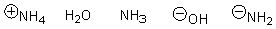
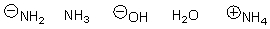
A)
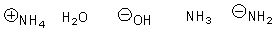
B)
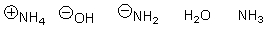
C)
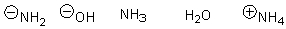
D)
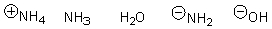
E)
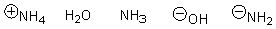

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
22
Predict the major product of the following reaction: 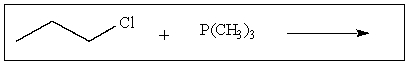
A)
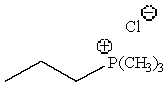
B)
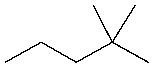
C)
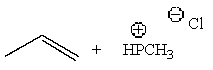
D)
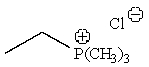
E) None of the above.

A)

B)

C)

D)

E) None of the above.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
23
What is the best way to prepare the compound below? 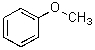
A)
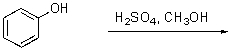
B)
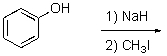
C)
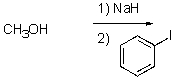
D)
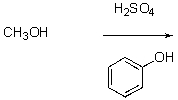
E) None of the above.

A)
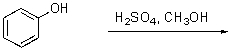
B)
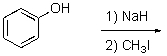
C)
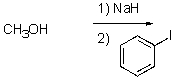
D)

E) None of the above.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
24
Predict the major product of the following reaction: 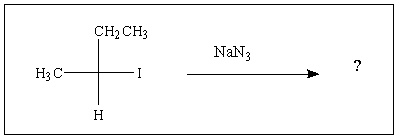
A)
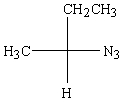
B)
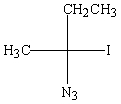
C)
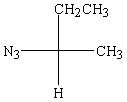
D)
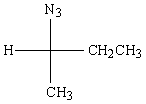
E) None of the above.

A)

B)

C)

D)

E) None of the above.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
25
Predict the major product of the following reaction: 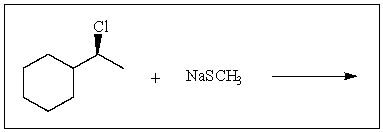
A)
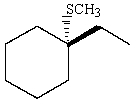
B)
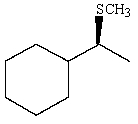
C)
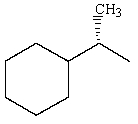
D)
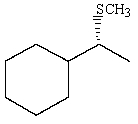
E) no reaction will occur

A)

B)

C)

D)

E) no reaction will occur

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
26
SN2 substitution at secondary halides and sulfonates is often complicated by competing E2 elimination.Which of the nucleophiles below would you choose to obtain the highest yield in an SN2 reaction with menthyl bromide? 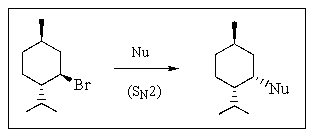
A) CH3ONa
B) CH3CO2Na
C) (CH3)3N
D) (CH3)3COK
E) C6H5SNa

A) CH3ONa
B) CH3CO2Na
C) (CH3)3N
D) (CH3)3COK
E) C6H5SNa

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
27
If the reaction rate of the following reaction is x,doubling the concentration of KCN would give what rate? 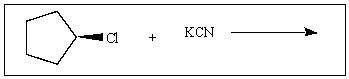
A) 2x
B) x/2
C) x2
D) x2/2
E) no change in reaction rate

A) 2x
B) x/2
C) x2
D) x2/2
E) no change in reaction rate

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
28
What is the correct stereochemistry of the product of the following reaction: 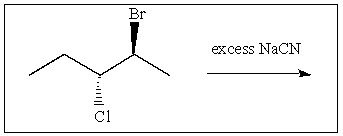
A) 3R,4S
B) 2S,3R
C) 2R,3S
D) 2R,3R
E) 3R,4R

A) 3R,4S
B) 2S,3R
C) 2R,3S
D) 2R,3R
E) 3R,4R

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
29
Name the following compound: 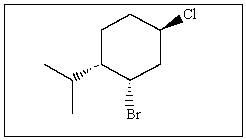
A) (1S,2S,5R)-1-bromo-2-isopropyl-5-chlorocyclohexane
B) (1R,2R,5R)-1-bromo-2-isopropyl-5-chlorocyclohexane
C) (1S,3S,4R)-1-chloro-3-bromo-4-isopropylcyclohexane
D) (1S,2S,4R)-2-bromo-4-chloro-1-isopropylcyclohexane
E) (1R,2S,4R)-2-bromo-4-chloro-1-isopropylcyclohexane

A) (1S,2S,5R)-1-bromo-2-isopropyl-5-chlorocyclohexane
B) (1R,2R,5R)-1-bromo-2-isopropyl-5-chlorocyclohexane
C) (1S,3S,4R)-1-chloro-3-bromo-4-isopropylcyclohexane
D) (1S,2S,4R)-2-bromo-4-chloro-1-isopropylcyclohexane
E) (1R,2S,4R)-2-bromo-4-chloro-1-isopropylcyclohexane

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
30
The curved arrows below represent what type of reaction mechanism? 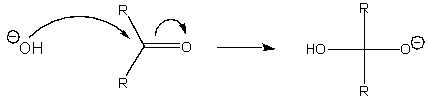
A) nucleophilic substitution
B) dissociation
C) nucleophilic addition
D) electrophilic addition
E) None of the above.

A) nucleophilic substitution
B) dissociation
C) nucleophilic addition
D) electrophilic addition
E) None of the above.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following can be used to synthesize (R)-2-cyanopentane from (R)-2-bromopentane?
A) NaBr
B) NaCN
C) NaI followed by KCN
D) NaCN followed by HI
E) this reaction cannot occur
A) NaBr
B) NaCN
C) NaI followed by KCN
D) NaCN followed by HI
E) this reaction cannot occur

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
32
The Walden Inversion (inversion of configuration)is associated with which of the following?
A) E1 reaction
B) free-radical halogenation
C) SN1 reaction
D) SN2 reaction
E) None of the above.
A) E1 reaction
B) free-radical halogenation
C) SN1 reaction
D) SN2 reaction
E) None of the above.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
33
Which set of reagents will best accomplish the following reaction? 
A) NaBr,acetone
B) H2SO4,H2O
C) Br2,hv
D) NaOEt,DMSO
E) None of the above.

A) NaBr,acetone
B) H2SO4,H2O
C) Br2,hv
D) NaOEt,DMSO
E) None of the above.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following statements are true of an SN2 reaction.
A) follow a first order rate law
B) typically are stereoselective
C) the fastest step is the rate-determining step
D) the carbocation intermediate adopts a trigonal planar geometry
E) all of the above
A) follow a first order rate law
B) typically are stereoselective
C) the fastest step is the rate-determining step
D) the carbocation intermediate adopts a trigonal planar geometry
E) all of the above

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
35
For the following reaction what is the most likely product? 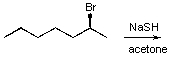
A)
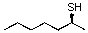
B)
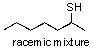
C)

D)

E) no reaction

A)

B)
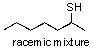
C)

D)

E) no reaction

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the haloalkanes shown below would react most rapidly with cyanide ion? 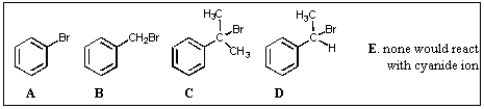
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is the best leaving group?
A)
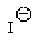
B)
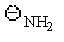
C)
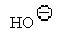
D)
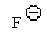
E)
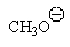
A)
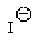
B)
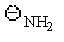
C)
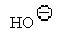
D)
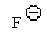
E)
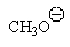

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following statements best describes why I- is a better nucleophile than F- in solution?
A) F- is a stronger base than I-.
B) Solvation of F- impedes its nucleophilicity compared to I-.
C) I- is a stronger base than F-.
D) Solvation of I- increases its nucleophilicity compared to that of F-.
E) A and B both offer explanations as to why I- is a better nucleophile than F- in solution.
A) F- is a stronger base than I-.
B) Solvation of F- impedes its nucleophilicity compared to I-.
C) I- is a stronger base than F-.
D) Solvation of I- increases its nucleophilicity compared to that of F-.
E) A and B both offer explanations as to why I- is a better nucleophile than F- in solution.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck


