Deck 7: Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/25
Play
Full screen (f)
Deck 7: Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
1
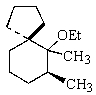
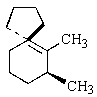
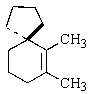
Indicate the expected major product of the following reaction: 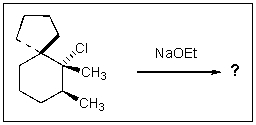
A)
B)
C)
D)
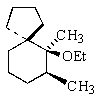
E)
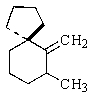

A)

B)

C)

D)

E)


2
Which of the cyclohexyl bromides would you expect to react the fastest in the following reaction? 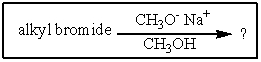
A)
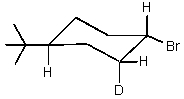
B)
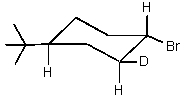
C)
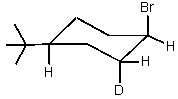
D)
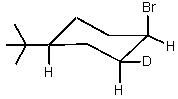
E) All would react at the same rate.

A)

B)

C)

D)

E) All would react at the same rate.

3
Which of the following molecules will readily undergo an elimination reaction when treated with NaOCH3?
A)
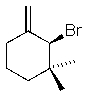
B)
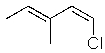
C)
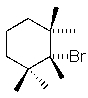
D)
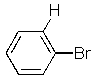
E) None of the above.
A)

B)

C)

D)

E) None of the above.

4
Which would be true of the following reactions? 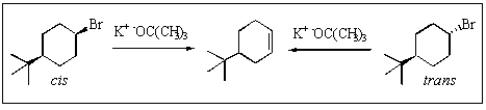
A) cis would react faster
B) trans would react faster
C) cis and trans would react at the same rates
D) no reaction is expected under these conditions
E) the product shown would not be formed

A) cis would react faster
B) trans would react faster
C) cis and trans would react at the same rates
D) no reaction is expected under these conditions
E) the product shown would not be formed

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the bases below would be best to accomplish the following reaction? 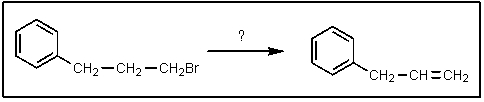
A) CH3O- Na+
B) CH3CH2O- Na+
C) (CH3)2CHO- Na+
D) (CH3)3CO- Na+
E) Na+ -OH

A) CH3O- Na+
B) CH3CH2O- Na+
C) (CH3)2CHO- Na+
D) (CH3)3CO- Na+
E) Na+ -OH

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
6
(S)-1-bromo-1-fluoroethane reacts with NaOMe to give: 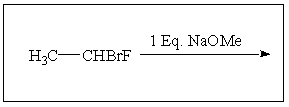
A)
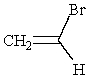
B)
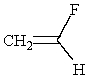
C) CH3CHFOCH3
D) CH3CHBrOCH3
E) CH3CH(OCH3)2

A)

B)

C) CH3CHFOCH3
D) CH3CHBrOCH3
E) CH3CH(OCH3)2

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is the least nucleophilic base?
A) (CH3CH2)3N
B)
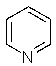
C)
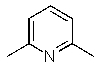
D)
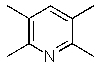
E)
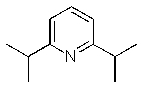
A) (CH3CH2)3N
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
8
Which mechanism proceeds with inversion of configuration?
A) bimolecular elimination (E2)
B) unimolecular elimination (E1)
C) unimolecular substitution (SN1)
D) bimolecular substitution (SN2)
E) free-radical halogenation
A) bimolecular elimination (E2)
B) unimolecular elimination (E1)
C) unimolecular substitution (SN1)
D) bimolecular substitution (SN2)
E) free-radical halogenation

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
9
Predict the major product of the following reaction: 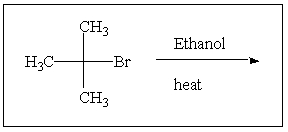
A)
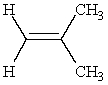 only
only
B)
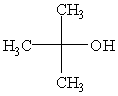
C)
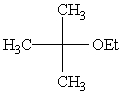 only
only
D)
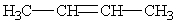
E)
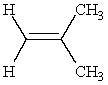 and
and
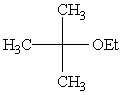

A)
 only
onlyB)

C)
 only
onlyD)
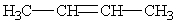
E)
 and
and

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
10
How many alkene products are possible in the following reaction? 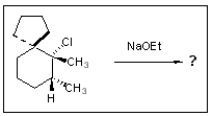
A) None; cannot eliminate
B) One
C) Two
D) Three
E) Four

A) None; cannot eliminate
B) One
C) Two
D) Three
E) Four

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
11
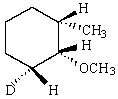
Predict the product of reaction of the following deuterated compound. 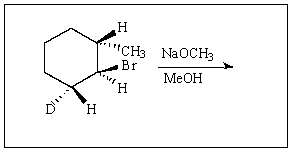
A)
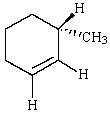
B)
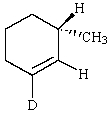
C)
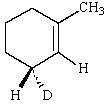
D)
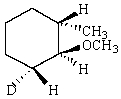
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
12
To which side,if any,would the following equilibrium lie? (Do not consider any further reactions here.) 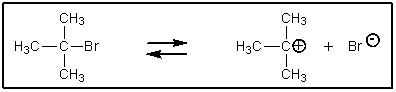
A) to the left
B) to the right
C) equally to the right and left
D) there is no way to tell
E) this reaction cannot occur at all

A) to the left
B) to the right
C) equally to the right and left
D) there is no way to tell
E) this reaction cannot occur at all

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
13
The major product of the following reaction conditions will result from: 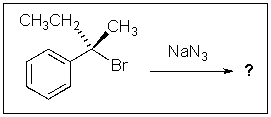
A) SN2
B) SN1
C) E2
D) E1
E) there is no way to know

A) SN2
B) SN1
C) E2
D) E1
E) there is no way to know

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
14
What would be the major organic product of the following reaction? 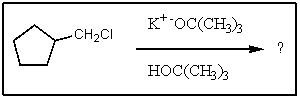
A)
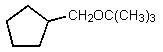
B)
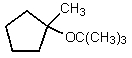
C)
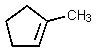
D)
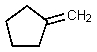
E)
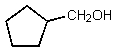

A)
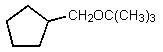
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
15
Solvolysis of 2-bromo-2-methylpropane occurs through what mechanism?
A) SN2
B) E1
C) E2
D) SN1
E) A and C
A) SN2
B) E1
C) E2
D) SN1
E) A and C

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
16
If the concentration of NaOH is doubled in the following reaction,what will happen to the reaction rate? 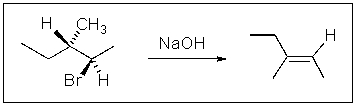
A) no change
B) double
C) quadruple
D) cut in half
E) None of the above.

A) no change
B) double
C) quadruple
D) cut in half
E) None of the above.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the haloalkanes below would you expect to most rapidly undergo the reaction shown? 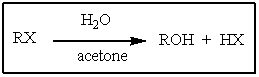
A) CH3CH2Br
B) CH3Br
C) (CH3)3CBr
D) CH3CHBrCH3
E)
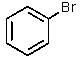

A) CH3CH2Br
B) CH3Br
C) (CH3)3CBr
D) CH3CHBrCH3
E)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
18
Which one of the following would undergo E2 elimination most rapidly?
A)
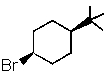
B)
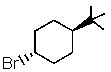
C)
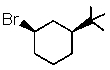
D)
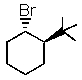
E) All would react at the same rate.
A)

B)

C)

D)

E) All would react at the same rate.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
19
Predict the major product of the following SN1 reaction: 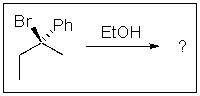
A)
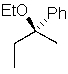
B)
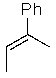
C)
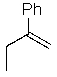
D)
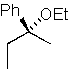
E)
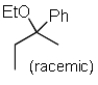

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the nucleophiles shown below would not cause an E2 elimination as the predominant reaction? 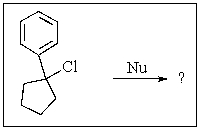
A) NaNH2
B) CH3CH2OH
C) (-CN)
D) (-OH)
E) CH3O-

A) NaNH2
B) CH3CH2OH
C) (-CN)
D) (-OH)
E) CH3O-

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
21
Rank the following carbocations according to their increasing stability. 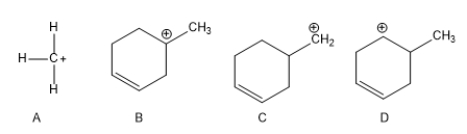
A) AB) AC) CD) AE) None of the above.

A) A

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
22
For the transformation of norepinephrine to epinephrine (adrenaline),what is the likely mechanism? 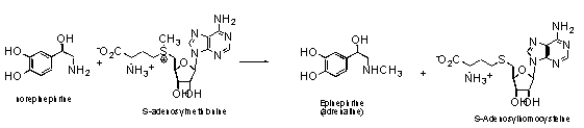
A) SN1
B) SN2
C) E1
D) E2
E) both A and B are likely

A) SN1
B) SN2
C) E1
D) E2
E) both A and B are likely

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
23
For the transformation below,give the structure of the starting material. 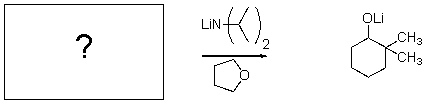
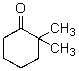
A)
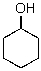
B)
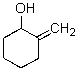
C)
D)
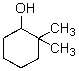
E)
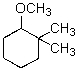

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
24
For the transformation below,give the structure of the starting material. 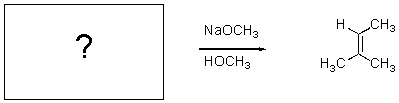
A)
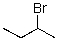
B)
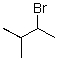
C)
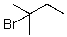
D)
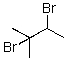
E) Both B and C will provide the product shown.

A)

B)

C)

D)

E) Both B and C will provide the product shown.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
25
Rank the following according to their increasing reactivity with water. 
A) CH3Br < CH3CH2Br < (CH3)3CBr < ( CH3)2CHBr
B) CH3Br < CH3CH2Br < ( CH3)2CHBr < (CH3)3CBr
C) (CH3)3CBr < ( CH3)2CHBr < CH3CH2Br < CH3Br
D) All of these will react with water at the same rate.
E) None of the above will react with water.

A) CH3Br < CH3CH2Br < (CH3)3CBr < ( CH3)2CHBr
B) CH3Br < CH3CH2Br < ( CH3)2CHBr < (CH3)3CBr
C) (CH3)3CBr < ( CH3)2CHBr < CH3CH2Br < CH3Br
D) All of these will react with water at the same rate.
E) None of the above will react with water.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck


