Deck 4: Cycloalkanes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/30
Play
Full screen (f)
Deck 4: Cycloalkanes
1
Which of the following compounds has the highest heat of combustion per CH2 group?
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
E) All have equal Hcombustion.
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
E) All have equal Hcombustion.
cyclopropane
2
The most stable conformation of cis-1,3-dimethylcyclohexane has how many hydrogen atoms in axial positions?
A) 4
B) 5
C) 6
D) 8
E) None of the above.
A) 4
B) 5
C) 6
D) 8
E) None of the above.
6
3
Which of the following correctly shows the Newman projection along a C-C bond in cyclohexane? (the squiggles indicate where the rest of the ring is attached) 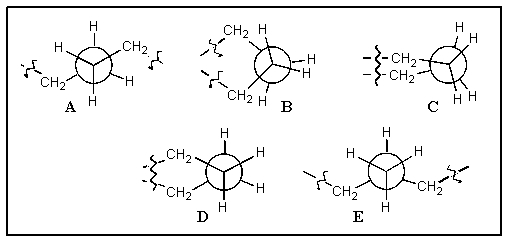
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E
D
4
Which of the following statements about conformations of methylcyclohexane is true? 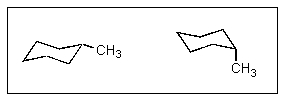
A) The energy barrier to interconvert these is too high to be achieved at room temperature.
B) The two forms are in equilibrium and are present in equal amounts at room temperature.
C) The two forms are not in equilibrium but are present in equal amounts at room temperature.
D) The two forms are in equilibrium but are not present in equal amounts at room temperature.
E) The two forms are not in equilibrium and are not present in equal amounts at room temperature.

A) The energy barrier to interconvert these is too high to be achieved at room temperature.
B) The two forms are in equilibrium and are present in equal amounts at room temperature.
C) The two forms are not in equilibrium but are present in equal amounts at room temperature.
D) The two forms are in equilibrium but are not present in equal amounts at room temperature.
E) The two forms are not in equilibrium and are not present in equal amounts at room temperature.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following isomers would you expect to have the lowest heat of combustion? (i.e.,be most stable)
A) cis-1,2-dimethylcyclohexane
B) trans-1,3-dimethylcyclohexane
C) cis-1,4-dimethylcyclohexane
D) cis-1,3-dimethylcyclohexane
E) All should have the same heat of combustion.
A) cis-1,2-dimethylcyclohexane
B) trans-1,3-dimethylcyclohexane
C) cis-1,4-dimethylcyclohexane
D) cis-1,3-dimethylcyclohexane
E) All should have the same heat of combustion.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following cyclic alkanes can be ring-opened under hydrogenation conditions? 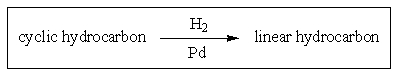
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
E) more than one of these

A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
E) more than one of these

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following compounds has the lowest heat of combustion per CH2 group?
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
E) All have equal Hcombustion.
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
E) All have equal Hcombustion.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
8
Which one of the following cyclic alkanes has the greatest tendency to have a planar ring?
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
E) None of the above are planar.
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
E) None of the above are planar.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
9
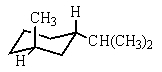
Which would be the most stable conformation of trans-1-methyl-3-isopropylcyclohexane?
A)
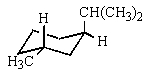
B)
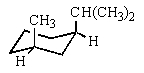
C)
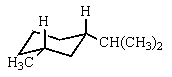
D)
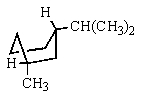
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
10
Which,if either,of the two isomers of the compound shown below would be more stable? 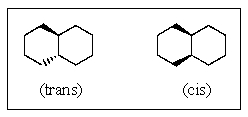
A) Cis is more stable.
B) Trans is more stable.
C) Both are equally stable.
D) Neither is stable.
E) There is no way to predict this.

A) Cis is more stable.
B) Trans is more stable.
C) Both are equally stable.
D) Neither is stable.
E) There is no way to predict this.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following disubstituted cyclohexanes could exist in a conformation that has both groups equatorial?
A) cis-1,3-dimethylcyclohexane
B) cis-1,4-dimethylcyclohexane
C) trans-1,3-dimethylcyclohexane
D) cis-1,2-dimethylcyclohexane
E) All or none can have both groups equatorial.
A) cis-1,3-dimethylcyclohexane
B) cis-1,4-dimethylcyclohexane
C) trans-1,3-dimethylcyclohexane
D) cis-1,2-dimethylcyclohexane
E) All or none can have both groups equatorial.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
12
What would be the name of the following? 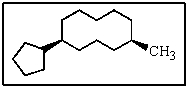
A) 5-cyclopentyl-1-methylcyclononane
B) cis-1-cyclopentyl-5-methylcyclodecane
C) cis-5-methyl-1-cyclopentylcyclododecane
D) trans-cyclopentyl-5-methylcyclodecane
E) (5-methylcyclodecyl)cyclopentane

A) 5-cyclopentyl-1-methylcyclononane
B) cis-1-cyclopentyl-5-methylcyclodecane
C) cis-5-methyl-1-cyclopentylcyclododecane
D) trans-cyclopentyl-5-methylcyclodecane
E) (5-methylcyclodecyl)cyclopentane

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
13
Terpenes can be considered to be built up from what units?
A)
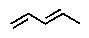
B)
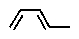
C)
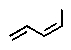
D)
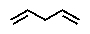
E)
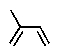
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following ring systems belongs to the class of compounds called steroids?
A)
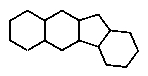
B)
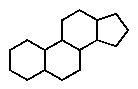
C)
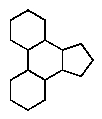
D)
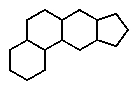
E)
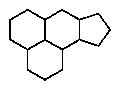
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
15
What is the correct IUPAC name for the following molecule: 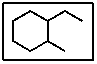
A) 1-ethyl-2-methylhexane
B) 2-ethyl-1-methylcycloheptane
C) 4-ethyl-5-methylcyclohexane
D) 1-ethyl-2-methylcyclohexane
E) 1-methyloctane

A) 1-ethyl-2-methylhexane
B) 2-ethyl-1-methylcycloheptane
C) 4-ethyl-5-methylcyclohexane
D) 1-ethyl-2-methylcyclohexane
E) 1-methyloctane

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following correctly represents cyclopropylcyclohexane?
A)
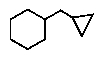
B)
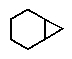
C)
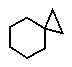
D)
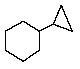
E) None of the above.
A)

B)

C)

D)

E) None of the above.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
17
Although five- and six-membered rings are generally the most stable,why is cyclopentane less stable than cyclohexane?
A) The angles in cyclopentane deviate significantly from the tetrahedral angle.
B) Five-membered rings have trans annular interactions.
C) Five-membered rings have eclipsing hydrogens.
D) Planar cyclohexane has bond angles closer to 109.
E) Larger rings are always more stable than smaller rings.
A) The angles in cyclopentane deviate significantly from the tetrahedral angle.
B) Five-membered rings have trans annular interactions.
C) Five-membered rings have eclipsing hydrogens.
D) Planar cyclohexane has bond angles closer to 109.
E) Larger rings are always more stable than smaller rings.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following could have both methyl groups in an equatorial orientation?
A)
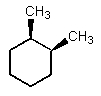
B)
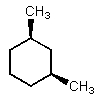
C)
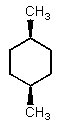
D)
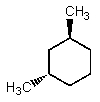
E)
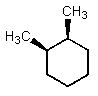
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following could be classified as a terpene?
A)
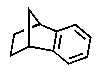
B)
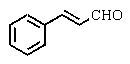
C)
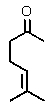
D)
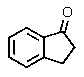
E)
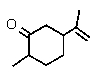
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
20
What would be the proper name of the following: 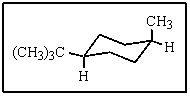
A) cis-1-tert-butyl-4-methylcyclohexane
B) trans-1-tert-butyl-4-methylcyclohexane
C) axial,equatorial-1-tert-butyl-4-methylcyclohexane
D) cis-1-isopropyl-4-methylcyclohexane
E) trans-1-isopropyl-4-methylcyclohexane

A) cis-1-tert-butyl-4-methylcyclohexane
B) trans-1-tert-butyl-4-methylcyclohexane
C) axial,equatorial-1-tert-butyl-4-methylcyclohexane
D) cis-1-isopropyl-4-methylcyclohexane
E) trans-1-isopropyl-4-methylcyclohexane

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following structures represent cis-1,4-dimethylcyclohexane? 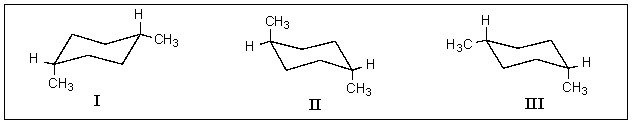
A) I & II
B) I & III
C) II & III
D) All of the above.
E) None of the above.

A) I & II
B) I & III
C) II & III
D) All of the above.
E) None of the above.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
22
How many isoprene units are in eudesmal? 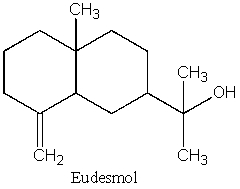
A) 0
B) 1
C) 2
D) 3
E) Unable to determine

A) 0
B) 1
C) 2
D) 3
E) Unable to determine

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
23
What is the correct structure of 2-methyl-1,3-butadiene?
A)
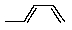
B)
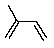
C)
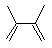
D)
E) None of the above.
A)

B)

C)

D)

E) None of the above.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
24
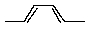
Which conformation of cyclohexane experiences the most transannular strain?
A) Chair
B) Planar
C) Boat
D) Twist boat
E) All of these are stable.
A) Chair
B) Planar
C) Boat
D) Twist boat
E) All of these are stable.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
25
What is the most stable conformation of trans-1-fluoro-4-methylcyclohexane?
A)

B)
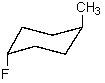
C)

D)

E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
26
Steroids frequently function as _____________,which are regulators of biochemical activity.
A) proteins
B) nucleic acids
C) hormones
D) fatty acids
E) triglycerides
A) proteins
B) nucleic acids
C) hormones
D) fatty acids
E) triglycerides

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is not in its most stable conformation?
A)
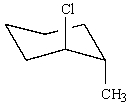
B)
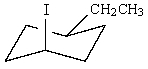
C)
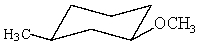
D)
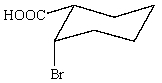
E) All of these are in their most stable conformation.
A)

B)

C)

D)

E) All of these are in their most stable conformation.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
28
What is the correct name for the following molecule? 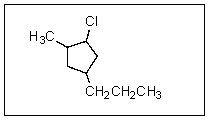
A) 1-chloro-2-methyl-4-propylcyclopentane
B) 2-chloro-1-methyl-4-propylcyclopentane
C) 1-chloro-5-methyl-3-propylcyclopentane
D) 5-methyl-1-chloro-3-propylcylopentane
E) 1-chloro-3-propyl-5-methylcyclopentane

A) 1-chloro-2-methyl-4-propylcyclopentane
B) 2-chloro-1-methyl-4-propylcyclopentane
C) 1-chloro-5-methyl-3-propylcyclopentane
D) 5-methyl-1-chloro-3-propylcylopentane
E) 1-chloro-3-propyl-5-methylcyclopentane

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
29
What is the potential energy change to convert from a twist-boat to boat conformation?
A) -14 Kcal/mol
B) 0 Kcal/mol
C) 1.4 Kcal/mol
D) 14 Kcal/mol
E) 45 Kcal/mol
A) -14 Kcal/mol
B) 0 Kcal/mol
C) 1.4 Kcal/mol
D) 14 Kcal/mol
E) 45 Kcal/mol

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
30
Cyclohexanes exhibit a higher_______than their straight-chain analogs.(Choose the correct answer)
A) boiling point
B) melting point
C) density
D) All of these are correct.
E) Two of these are correct.
A) boiling point
B) melting point
C) density
D) All of these are correct.
E) Two of these are correct.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck


