Deck 5: Stereoisomers
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/44
Play
Full screen (f)
Deck 5: Stereoisomers
1
Which of the following molecules have the S configuration? 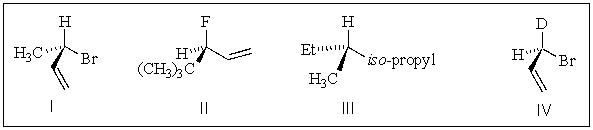
A) I,II
B) I,III
C) III,IV
D) I,II,IV
E) All of the above.

A) I,II
B) I,III
C) III,IV
D) I,II,IV
E) All of the above.
I,II
2
How many stereogenic (chiral)centers are found in Rhizoxin? 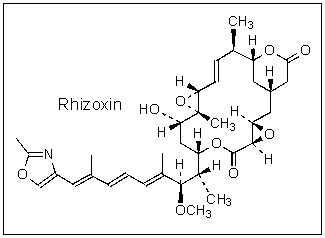
A) 5
B) 7
C) 9
D) 11
E) 14

A) 5
B) 7
C) 9
D) 11
E) 14
11
3
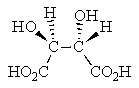
Which of the following Fischer projections represents (2R,3R)-tartaric acid?
A)
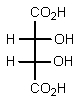
B)
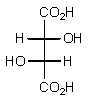
C)
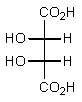
D)
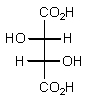
E) None of the above.
A)

B)

C)

D)

E) None of the above.

4
What (R)or (S)stereochemistry is proper for the following molecule? 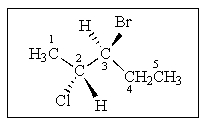
A) (2R,3R)
B) (2R,3S)
C) (2S,3S)
D) (2S,3R)
E) None of the above.

A) (2R,3R)
B) (2R,3S)
C) (2S,3S)
D) (2S,3R)
E) None of the above.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
5
The meso isomer of 3,4-dibromohexane has what stereochemical configuration?
A) 3R,4S
B) 3R,4R
C) 3S,4S
D) 3S,4R
E) both A and D
A) 3R,4S
B) 3R,4R
C) 3S,4S
D) 3S,4R
E) both A and D

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
6
How many total stereoisomers of the following are possible? 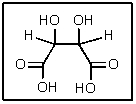
A) 1
B) 2
C) 3
D) 4
E) 6

A) 1
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
7
How many total stereoisomers of the following are possible? 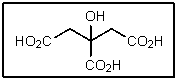
A) 1
B) 2
C) 3
D) 4
E) 6

A) 1
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
8
How many chiral centers are present in -cadinene? 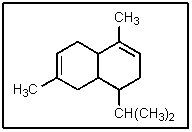
A) none
B) 1
C) 2
D) 3
E) 4

A) none
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
9
How many total stereoisomers of the following are possible? 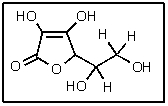
A) 1
B) 2
C) 3
D) 4
E) 6

A) 1
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
10
(S)-Naproxen,[]D = + 66,is an analgesic (pain reliever),while its enantiomer is toxic.Say that you were given a solution that contains 1 g of Naproxen in 20 mL of liquid,but the optical purity is not specified.You place it in a polarimeter tube (10 cm)and get a reading of + 3.3 from the polarimeter.What is the percent optical purity of the sample?
A) 50
B) 100
C) 0
D) 10
E) 75
A) 50
B) 100
C) 0
D) 10
E) 75

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
11
The correct structure for (R)-bromofluoroiodomethane is:
A)
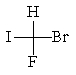
B)
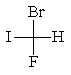
C)
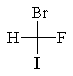
D)
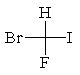
E)
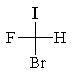
A)

B)

C)

D)

E)
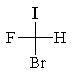

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
12
Counting all stereoisomers,how many monochlorinated products of the free-radical chlorination of (R)-2-bromobutane are possible? (Note that the starting compound is one enantiomer only.) 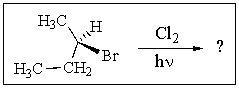
A) 3
B) 4
C) 5
D) 6
E) 8

A) 3
B) 4
C) 5
D) 6
E) 8

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
13
The following molecule has how many possible stereoisomers? 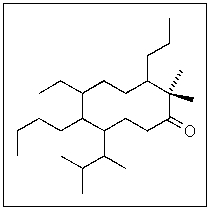
A) 1
B) 4
C) 8
D) 16
E) 32

A) 1
B) 4
C) 8
D) 16
E) 32

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
14
What would be the complete name of the following? 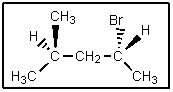
A) (2R,4S)-2-bromopentane
B) (R)-2-bromo-4-methylpentane
C) (S)-4-bromo-2-methylpentane
D) (2R,4R)-2-bromo-4-methylpentane
E) (S)-2-bromo-4-methylpentane

A) (2R,4S)-2-bromopentane
B) (R)-2-bromo-4-methylpentane
C) (S)-4-bromo-2-methylpentane
D) (2R,4R)-2-bromo-4-methylpentane
E) (S)-2-bromo-4-methylpentane

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the amines below might be appropriate for the resolution of racemic Ibuprofen? 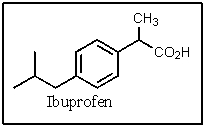
A)
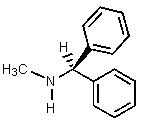
B)
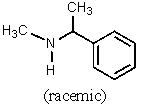
C)
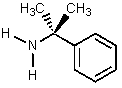
D)
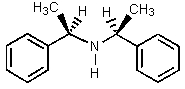
E)
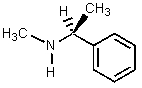

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
16
The relationship between the following two compounds is: 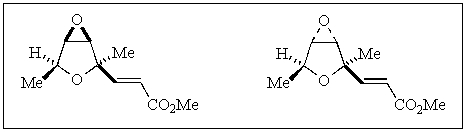
A) same molecule
B) enantiomers
C) diastereomers
D) mesos
E) conformers

A) same molecule
B) enantiomers
C) diastereomers
D) mesos
E) conformers

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
17
A particular reaction produces the following two alcohols in a ratio of 95:5. 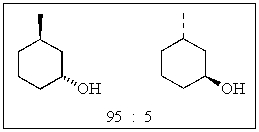 The enantiomeric excess (% ee)is:
The enantiomeric excess (% ee)is:
A) 100
B) 95
C) 90
D) 85
E) None of the above.
 The enantiomeric excess (% ee)is:
The enantiomeric excess (% ee)is:A) 100
B) 95
C) 90
D) 85
E) None of the above.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
18
The following two molecules may be described as: 
A) constitutional isomers
B) diastereomers
C) enantiomers
D) structural isomers
E) None of the above.

A) constitutional isomers
B) diastereomers
C) enantiomers
D) structural isomers
E) None of the above.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
19
Optically pure (S)-monosodium glutamate has a specific rotation of + 24.What specific rotation would (R)-monosodium glutamate of 50% optical purity have?
A) + 24
B) - 24
C) - 18
D) - 12
E) + 18
A) + 24
B) - 24
C) - 18
D) - 12
E) + 18

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements is not true?
A) Enantiomers have identical properties except in chiral environments or with plane-polarized light.
B) Reactions involving only achiral or racemic materials must produce achiral or racemic products.
C) Diastereomers have identical properties in all environments.
D) Enantiomers exhibit equal and opposite optical rotations.
E) All of the above are true.
A) Enantiomers have identical properties except in chiral environments or with plane-polarized light.
B) Reactions involving only achiral or racemic materials must produce achiral or racemic products.
C) Diastereomers have identical properties in all environments.
D) Enantiomers exhibit equal and opposite optical rotations.
E) All of the above are true.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
21
The best (most reliable)test for the presence of chirality in a molecule is
A) carbon attached to four different groups.
B) existance of a mirror image.
C) non-superimposability on mirror image.
D) two or more isomers possible.
E) observation of optical rotation in a sample.
A) carbon attached to four different groups.
B) existance of a mirror image.
C) non-superimposability on mirror image.
D) two or more isomers possible.
E) observation of optical rotation in a sample.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
22
What technique(s)can be used to obtain non-racemic compounds from racemic material?
A) resolution
B) distillation
C) extraction
D) column chromatography
E) both B and C
A) resolution
B) distillation
C) extraction
D) column chromatography
E) both B and C

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
23
How are the following compounds related? 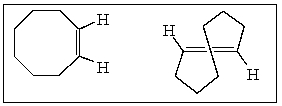
A) diastereomers
B) enantiomers
C) meso compounds
D) same compound
E) not related

A) diastereomers
B) enantiomers
C) meso compounds
D) same compound
E) not related

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
24
How many stereogenic (chiral)centers are present in the following molecule: 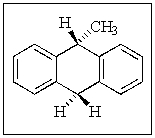
A) none
B) one
C) two
D) three
E) four

A) none
B) one
C) two
D) three
E) four

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
25
How are the following compounds related? 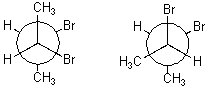
A) Diastereomers
B) Enantiomers
C) Meso compounds
D) Same compound
E) They are not related.
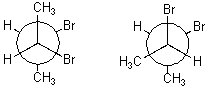
A) Diastereomers
B) Enantiomers
C) Meso compounds
D) Same compound
E) They are not related.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
26
A graduate student wishes to separate a racemic mixture of acids prepared in the laboratory as shown below.The best way(s)to accomplish this task is: 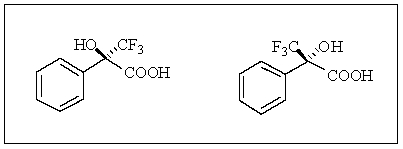
A) distillation.
B) water solubility.
C) reaction with a chiral amine to form diastereomers,then crystallization.
D) column chromatography.
E) both A and B

A) distillation.
B) water solubility.
C) reaction with a chiral amine to form diastereomers,then crystallization.
D) column chromatography.
E) both A and B

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
27
What is the correct structure for (R)- 4-methyl-2-heptanone?
A)
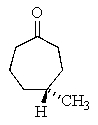
B)
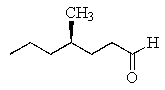
C)
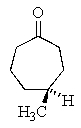
D)
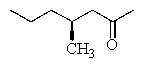
E)
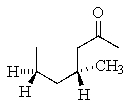
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
28
Penicillin V was discovered in the late 1920s by Sir Alexander Fleming in which he won the Nobel Prize for the development of this wonder drug.How many sterogenic centers does penicillin V contain? 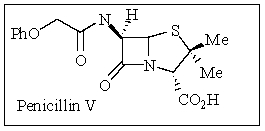
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the structures below are pairs of enantiomers? 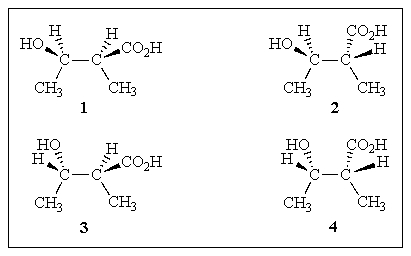
A) (1+2)and (3+4)
B) (1+4)and (2+3)
C) (1+3)and (2+4)
D) All of the above.
E) None of the above.

A) (1+2)and (3+4)
B) (1+4)and (2+3)
C) (1+3)and (2+4)
D) All of the above.
E) None of the above.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following molecules represents a meso compound?
A)
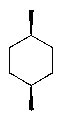
B)
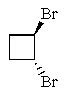
C)
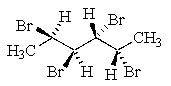
D)
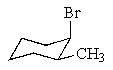
E)
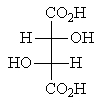
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
31
How would you most accurately describe the relationship between the following two molecules? 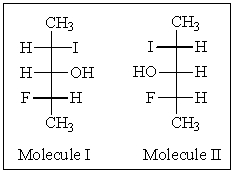
A) enantiomers
B) diastereomers
C) meso compounds
D) same compound
E) Both C and D

A) enantiomers
B) diastereomers
C) meso compounds
D) same compound
E) Both C and D

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
32
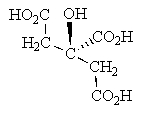
What is the R and S configuration for each stereogenic center of the following sugar from top to bottom? 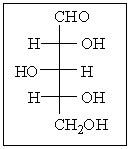
A) R,S,R
B) S,S,R
C) R,R,S
D) R,R,R
E) S,S,S

A) R,S,R
B) S,S,R
C) R,R,S
D) R,R,R
E) S,S,S

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
33
An unknown compound has been isolated in pure form and found to exhibit []D = + 15 (c = 4,CH2Cl2).Which of the following might be the structure of the compound?
A)
![<strong>An unknown compound has been isolated in pure form and found to exhibit [<font face=symbol></font>]<sub>D</sub> = + 15<font face=symbol></font> (c = 4,CH<sub>2</sub>Cl<sub>2</sub>).Which of the following might be the structure of the compound?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6928/11eab5f7_3d6f_d4af_a628_5157de30304c_TB6928_00.jpg)
B)
![<strong>An unknown compound has been isolated in pure form and found to exhibit [<font face=symbol></font>]<sub>D</sub> = + 15<font face=symbol></font> (c = 4,CH<sub>2</sub>Cl<sub>2</sub>).Which of the following might be the structure of the compound?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6928/11eab5f7_3d6f_d4b0_a628_a5aa8ecd99fd_TB6928_00.jpg)
C)
![<strong>An unknown compound has been isolated in pure form and found to exhibit [<font face=symbol></font>]<sub>D</sub> = + 15<font face=symbol></font> (c = 4,CH<sub>2</sub>Cl<sub>2</sub>).Which of the following might be the structure of the compound?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6928/11eab5f7_3d6f_fbc1_a628_e94f3cd5f7a6_TB6928_00.jpg)
D)
![<strong>An unknown compound has been isolated in pure form and found to exhibit [<font face=symbol></font>]<sub>D</sub> = + 15<font face=symbol></font> (c = 4,CH<sub>2</sub>Cl<sub>2</sub>).Which of the following might be the structure of the compound?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6928/11eab5f7_3d6f_fbc2_a628_4badee6758e7_TB6928_00.jpg)
E)
![<strong>An unknown compound has been isolated in pure form and found to exhibit [<font face=symbol></font>]<sub>D</sub> = + 15<font face=symbol></font> (c = 4,CH<sub>2</sub>Cl<sub>2</sub>).Which of the following might be the structure of the compound?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6928/11eab5f7_3d6f_fbc3_a628_05ba23493e6d_TB6928_00.jpg)
A)
![<strong>An unknown compound has been isolated in pure form and found to exhibit [<font face=symbol></font>]<sub>D</sub> = + 15<font face=symbol></font> (c = 4,CH<sub>2</sub>Cl<sub>2</sub>).Which of the following might be the structure of the compound?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6928/11eab5f7_3d6f_d4af_a628_5157de30304c_TB6928_00.jpg)
B)
![<strong>An unknown compound has been isolated in pure form and found to exhibit [<font face=symbol></font>]<sub>D</sub> = + 15<font face=symbol></font> (c = 4,CH<sub>2</sub>Cl<sub>2</sub>).Which of the following might be the structure of the compound?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6928/11eab5f7_3d6f_d4b0_a628_a5aa8ecd99fd_TB6928_00.jpg)
C)
![<strong>An unknown compound has been isolated in pure form and found to exhibit [<font face=symbol></font>]<sub>D</sub> = + 15<font face=symbol></font> (c = 4,CH<sub>2</sub>Cl<sub>2</sub>).Which of the following might be the structure of the compound?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6928/11eab5f7_3d6f_fbc1_a628_e94f3cd5f7a6_TB6928_00.jpg)
D)
![<strong>An unknown compound has been isolated in pure form and found to exhibit [<font face=symbol></font>]<sub>D</sub> = + 15<font face=symbol></font> (c = 4,CH<sub>2</sub>Cl<sub>2</sub>).Which of the following might be the structure of the compound?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6928/11eab5f7_3d6f_fbc2_a628_4badee6758e7_TB6928_00.jpg)
E)
![<strong>An unknown compound has been isolated in pure form and found to exhibit [<font face=symbol></font>]<sub>D</sub> = + 15<font face=symbol></font> (c = 4,CH<sub>2</sub>Cl<sub>2</sub>).Which of the following might be the structure of the compound?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6928/11eab5f7_3d6f_fbc3_a628_05ba23493e6d_TB6928_00.jpg)

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
34
The structure of (-)-geosmin is shown below.Which structure would be that of its enantiomer,(+)-geosmin? 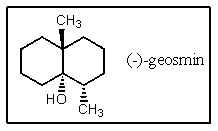
A)
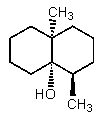
B)
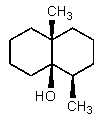
C)
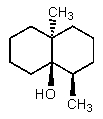
D)
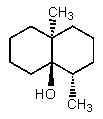
E) None of the above.

A)

B)

C)

D)

E) None of the above.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
35
Sharpless epoxidation of geraniol gave two products,epoxide I (85%)and epoxide II (15%).This mixture of epoxides represents what percent optical purity (or percent enantiomeric excess,% ee)? 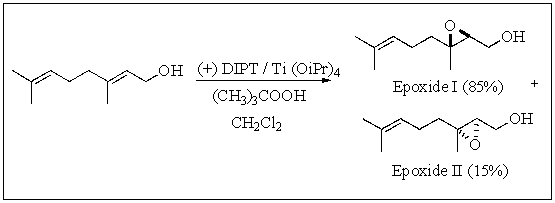
A) 0%
B) 15%
C) 70%
D) 85%
E) 100%

A) 0%
B) 15%
C) 70%
D) 85%
E) 100%

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the structures below are pairs of diastereomers? 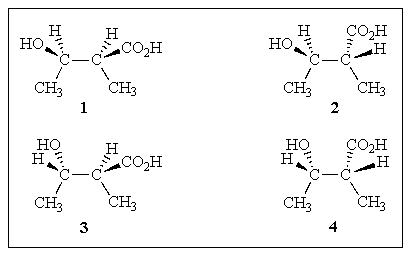
A) (1+4)and (2+3)
B) (1+3)and (2+4)
C) (1+2)and (3+4)
D) both A and B
E) both B and C

A) (1+4)and (2+3)
B) (1+3)and (2+4)
C) (1+2)and (3+4)
D) both A and B
E) both B and C

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is not true for a meso compound:
A) It is achiral.
B) It will rotate plane polarized light.
C) It may be cyclic or acyclic.
D) It is a stereoisomer.
E) It has a mirror plane.
A) It is achiral.
B) It will rotate plane polarized light.
C) It may be cyclic or acyclic.
D) It is a stereoisomer.
E) It has a mirror plane.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
38
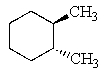
Which of the following molecules is not chiral?
A)
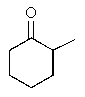
B)
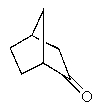
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
39
What would be the proper name of the following? 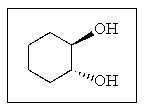
A) (1R,2R)-trans-1,2-cyclohexanediol
B) (1R,2S)-trans-1,2-cyclohexanediol
C) (1S,2R)-trans-1,2-cyclohexanediol
D) (1S,2S)-trans-1,2-cyclohexanediol
E) (1S,2R)-cis-1,2-cyclohexanediol

A) (1R,2R)-trans-1,2-cyclohexanediol
B) (1R,2S)-trans-1,2-cyclohexanediol
C) (1S,2R)-trans-1,2-cyclohexanediol
D) (1S,2S)-trans-1,2-cyclohexanediol
E) (1S,2R)-cis-1,2-cyclohexanediol

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
40
How are the following compounds related? 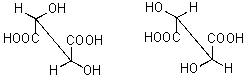
A) diastereomers
B) enantiomers
C) meso compounds
D) same compound
E) not related
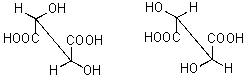
A) diastereomers
B) enantiomers
C) meso compounds
D) same compound
E) not related

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
41
Label the following carbons as either (R)or (S). 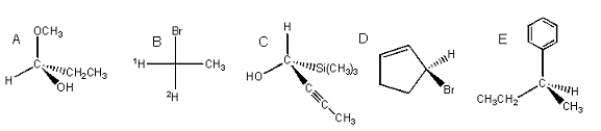
A) A = R,B = R,C = R,D = R,E = R
B) A = S,B = S,C = S,D = S,E = S
C) A = S,B = R,C = S,D = S,E = S
D) A = S,B = S,C = R,D = S,E = S
E) A = S,B = S,C = S,D = R,E = S

A) A = R,B = R,C = R,D = R,E = R
B) A = S,B = S,C = S,D = S,E = S
C) A = S,B = R,C = S,D = S,E = S
D) A = S,B = S,C = R,D = S,E = S
E) A = S,B = S,C = S,D = R,E = S

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
42
How are the following compounds related? 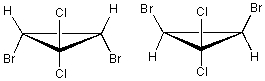
A) diastereomers
B) enantiomers
C) meso compounds
D) same compound
E) Both meso and the same compound

A) diastereomers
B) enantiomers
C) meso compounds
D) same compound
E) Both meso and the same compound

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
43
A racemic mixture will rotate light?
A) 0 degrees
B) 180 degrees
C) need more information
D) There is no such thing as a racemic mixture of enantiomers.
E) none of the above
A) 0 degrees
B) 180 degrees
C) need more information
D) There is no such thing as a racemic mixture of enantiomers.
E) none of the above

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
44
How are the following compounds related? 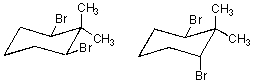
A) diastereomers
B) enantiomers
C) meso compounds
D) same compound
E) They are not related.

A) diastereomers
B) enantiomers
C) meso compounds
D) same compound
E) They are not related.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck


