Deck 23: Ester Enolates and the Claisen Condensation: Synthesis of B-Dicarbonyl Compounds; Acyl Anion Equivalents
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/29
Play
Full screen (f)
Deck 23: Ester Enolates and the Claisen Condensation: Synthesis of B-Dicarbonyl Compounds; Acyl Anion Equivalents
1
What missing reactant would be required to provide the product shown? 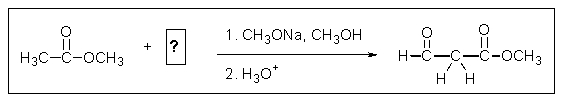
A)
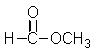
B)
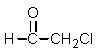
C)
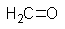
D)
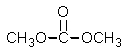
E) None of these would work.

A)
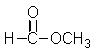
B)
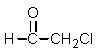
C)
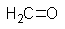
D)
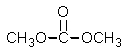
E) None of these would work.
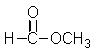
2
Which of the following would be the most acidic?
A)
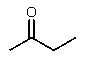
B) CH3CH2CO2CH3
C)
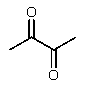
D)
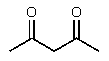
E)
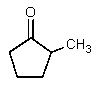
A)

B) CH3CH2CO2CH3
C)

D)

E)


3
What problem is encountered in the synthesis of ethyl acetoacetate (shown below)if sodium methoxide is used rather than sodium ethoxide? 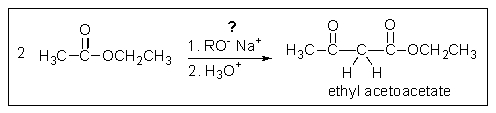
A) Sodium methoxide is too weak a base to cause this reaction to occur.
B) The condensation proceeds further to give tri-carbonyl products.
C) The reaction would produce some of both the methyl ester and the ethyl ester.
D) Sodium methoxide is too insoluble in methanol to promote the reaction.
E) Sodium methoxide is not basic enough to drive the reaction to completion.
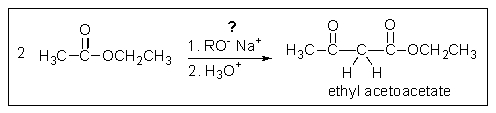
A) Sodium methoxide is too weak a base to cause this reaction to occur.
B) The condensation proceeds further to give tri-carbonyl products.
C) The reaction would produce some of both the methyl ester and the ethyl ester.
D) Sodium methoxide is too insoluble in methanol to promote the reaction.
E) Sodium methoxide is not basic enough to drive the reaction to completion.
The reaction would produce some of both the methyl ester and the ethyl ester.
4
Which of the following reaction sequences will affect the following transformation? 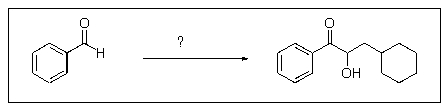
A)
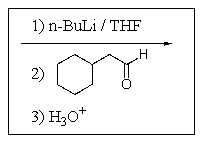
B)
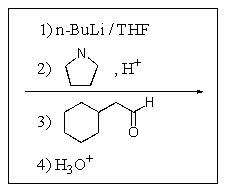
C)
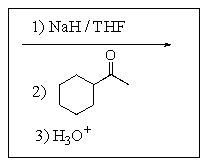
D)
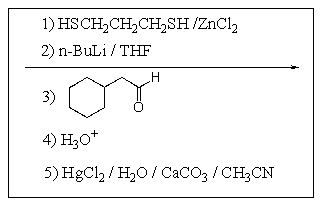
E)
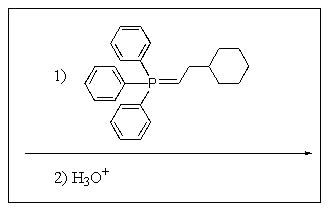

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
5
What product would be expected to result from the reaction sequence shown? 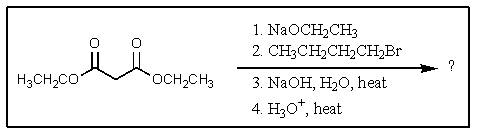
A)
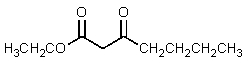
B)
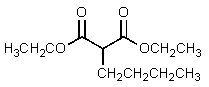
C)
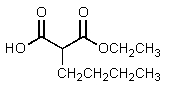
D)
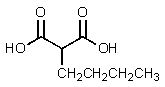
E)
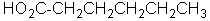

A)

B)

C)

D)

E)
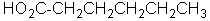

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
6
What would be the expected product of the following reaction sequence? 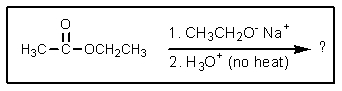
A)
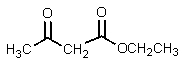
B)
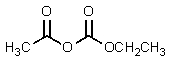
C)
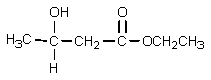
D)
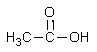
E)
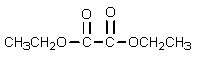

A)

B)

C)
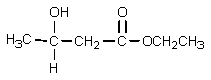
D)
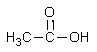
E)
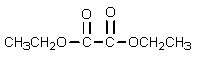

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
7
Predict the products of the following reaction. 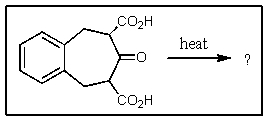
A)
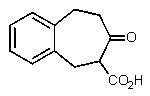
B)
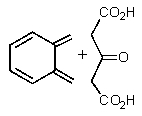
C)
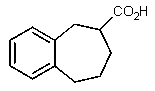
D)
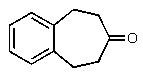
E) None of these.

A)

B)

C)

D)

E) None of these.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
8
Predict the major product of the following reaction? 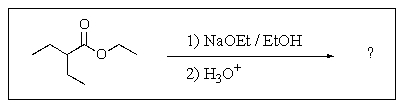
A)
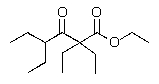
B)
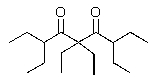
C)
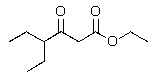
D)
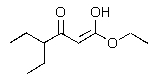
E) No significant reaction occurs.
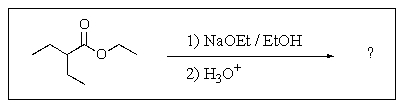
A)

B)

C)

D)

E) No significant reaction occurs.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
9
What factors contribute to the stability of the following structure? 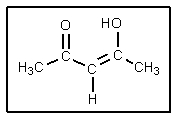
A) structure has a 6 electron aromatic system
B) stabilized by conjugation between C=C and C=O
C) C=C is generally more stable than C=O
D) structure has intramolecular hydrogen bonding
E) More than one of the above are true.

A) structure has a 6 electron aromatic system
B) stabilized by conjugation between C=C and C=O
C) C=C is generally more stable than C=O
D) structure has intramolecular hydrogen bonding
E) More than one of the above are true.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
10
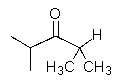
What would result from the reaction shown below? 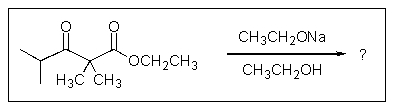
A)
B)
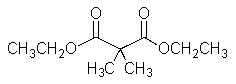
C)
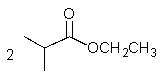
D)
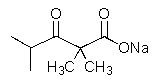
E)
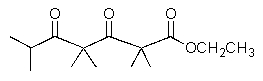

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
11
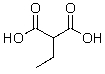
Which of the following compounds will not readily undergo decarboxylation (loss of CO2)upon heating in an appropriate solvent?
A)
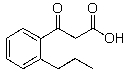
B)
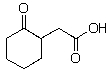
C)
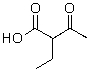
D)
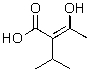
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
12
What product would result from the following reaction sequence? 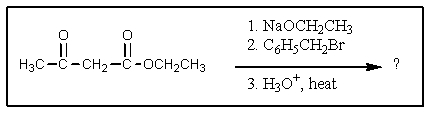
A)
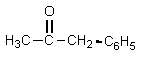
B)
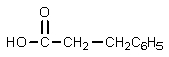
C)
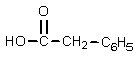
D)
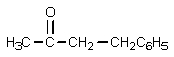
E)
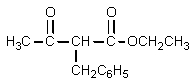

A)
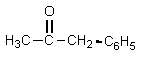
B)
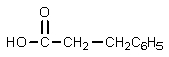
C)
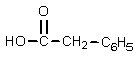
D)
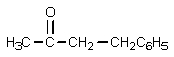
E)
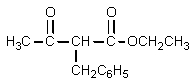

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
13
Predict the major organic product of the following reaction. 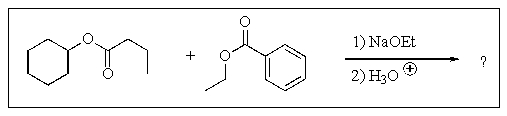
A)
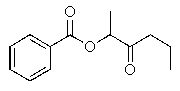
B)
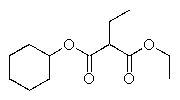
C)
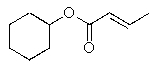
D)
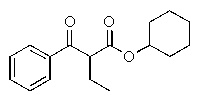
E) The reaction does not occur.

A)

B)

C)

D)

E) The reaction does not occur.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
14
Predict the major product of the following reaction. 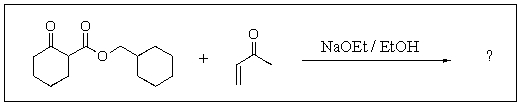
A)
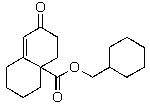
B)
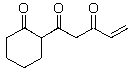
C)
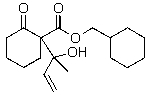
D)
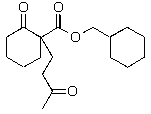
E)
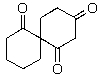

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
15
The Dieckmann reaction is best described by which of the following statements?
A) Intermolecular Aldol Condensation
B) Intramolecular Aldol Condensation
C) Intermolecular Claisen Condensation
D) Intramolecular Claisen Condensation
E) Michael Addition followed by intramolecular Aldol
A) Intermolecular Aldol Condensation
B) Intramolecular Aldol Condensation
C) Intermolecular Claisen Condensation
D) Intramolecular Claisen Condensation
E) Michael Addition followed by intramolecular Aldol

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
16
To which side,if any,would the following equilibrium lie? 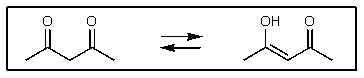
A) To the left
B) To the right
C) Equally to the left and right
D) Reaction cannot occur at all
E) Equilibrium favors a different product

A) To the left
B) To the right
C) Equally to the left and right
D) Reaction cannot occur at all
E) Equilibrium favors a different product

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
17
Why is the following reaction typically done? 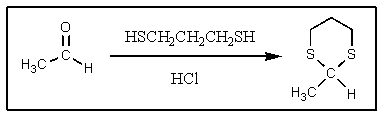
A) To protect the aldehyde against oxidation
B) To render the molecule inert to organometallic reagents
C) To make the aldehyde CH more acidic
D) To render the molecule more fragrant
E) To protect the aldehyde against reduction

A) To protect the aldehyde against oxidation
B) To render the molecule inert to organometallic reagents
C) To make the aldehyde CH more acidic
D) To render the molecule more fragrant
E) To protect the aldehyde against reduction

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
18
What would be the organic product of the following reaction? 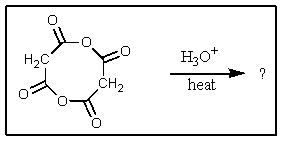
A) 2 HO2CCH2CO2H
B) 2 CH3C(=O)CH3
C) 2 CH3CO2H
D) HO2CCH2C(=O)O2CCH2C(=O)O2CCH2CO2H
E) No reaction occurs.

A) 2 HO2CCH2CO2H
B) 2 CH3C(=O)CH3
C) 2 CH3CO2H
D) HO2CCH2C(=O)O2CCH2C(=O)O2CCH2CO2H
E) No reaction occurs.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
19
Predict the major product of the following reaction. 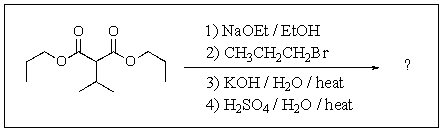
A)
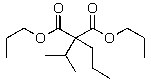
B)
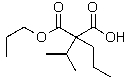
C)
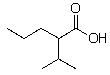
D)

E)
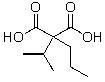

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
20
Predict the major organic product of the following reaction. 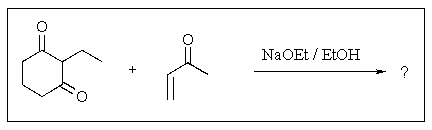
A)
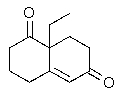
B)
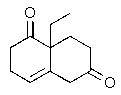
C)
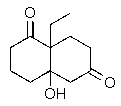
D)
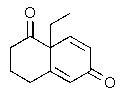
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following processes involves loss of one carbon as carbon dioxide?
A) Claisen condensation
B) Michael addition
C) Malonic ester synthesis
D) None involve loss of CO2.
E) All involve loss of CO2.
A) Claisen condensation
B) Michael addition
C) Malonic ester synthesis
D) None involve loss of CO2.
E) All involve loss of CO2.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the indicated hydrogens would be the most acidic? 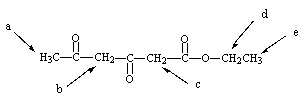
A) Hydrogens a
B) Hydrogens b
C) Hydrogens c
D) Hydrogens d
E) Hydrogens e

A) Hydrogens a
B) Hydrogens b
C) Hydrogens c
D) Hydrogens d
E) Hydrogens e

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
23
What product do you expect from the following reaction? 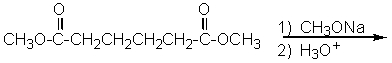
A)
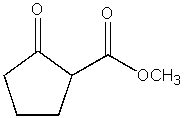
B)
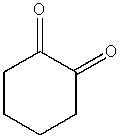
C)
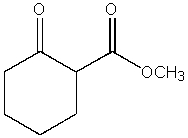
D)
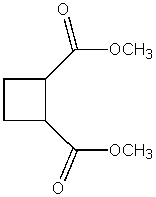
E)
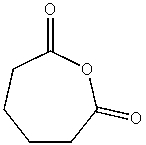
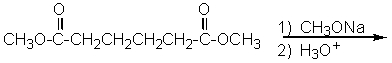
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
24
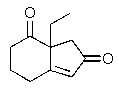
After treatment with aqueous base + heat,followed by acidification to pH 2 followed by heating,which carbon in the molecule below would be lost as carbon dioxide? 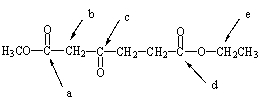
A) Carbon a
B) Carbon b
C) Carbon c
D) Carbon d
E) Carbon e

A) Carbon a
B) Carbon b
C) Carbon c
D) Carbon d
E) Carbon e

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
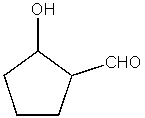
25
What product do you expect from the following reaction? 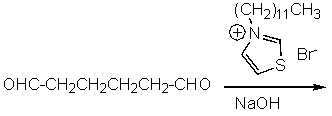
A)
B)
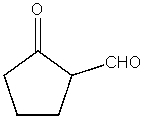
C)
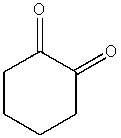
D)
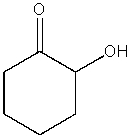
E) no reaction
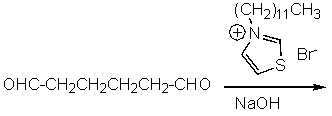
A)

B)

C)

D)

E) no reaction

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
26
What product do you expect from the following reaction? 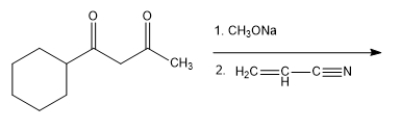
A)
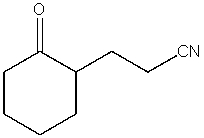
B)
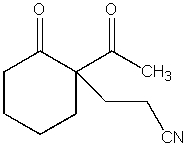
C)
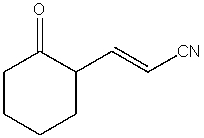
D)
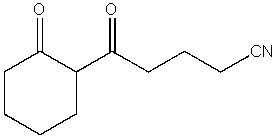
E)
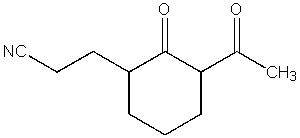

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
27
What product do you expect from the following reaction? 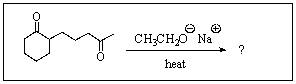
A)
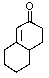
B)
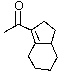
C)
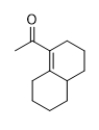
D) Some of these would be formed.
E) None of these would be formed.

A)

B)

C)

D) Some of these would be formed.
E) None of these would be formed.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
28
Which would be the most acidic proton in the molecule below? 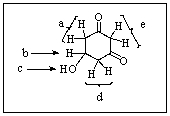
A) Protons a
B) Proton b
C) Proton c
D) Protons d
E) Protons e

A) Protons a
B) Proton b
C) Proton c
D) Protons d
E) Protons e

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
29
What would be the expected product of the following reaction sequence? 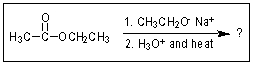
A)
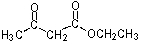
B)
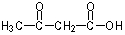
C)
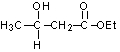
D)
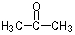
E)
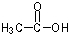

A)

B)
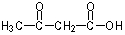
C)
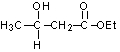
D)
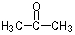
E)

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck


