Deck 17: Aldehydes and Ketones: the Carbonyl Group
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/32
Play
Full screen (f)
Deck 17: Aldehydes and Ketones: the Carbonyl Group
1
What would be the proper name of the following molecule? 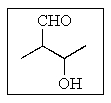
A) 3-formyl-2-butanol
B) 2-(hydroxyethyl)propanal
C) 3-hydroxy-2-methylbutanal
D) 2,3-dimethylpropan-3-ol-1-al
E) 1-methyl-3-formyl-1-propanol

A) 3-formyl-2-butanol
B) 2-(hydroxyethyl)propanal
C) 3-hydroxy-2-methylbutanal
D) 2,3-dimethylpropan-3-ol-1-al
E) 1-methyl-3-formyl-1-propanol
3-hydroxy-2-methylbutanal
2
What would be the major product of the following reaction sequence? 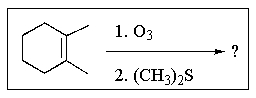
A)
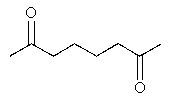
B)
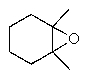
C)
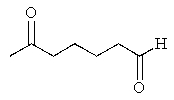
D)
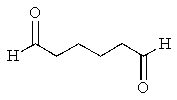
E)
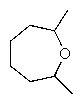

A)

B)

C)

D)

E)


3
What would be the expected product of the following reaction sequence? 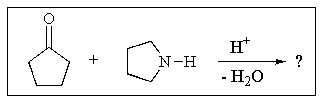
A)
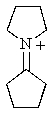
B)
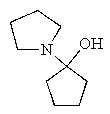
C)
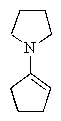
D)
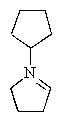
E)
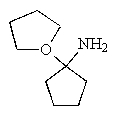

A)

B)

C)

D)

E)


4
What would you expect to result from the following reaction? 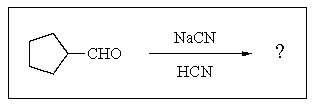
A)
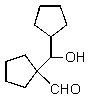
B)
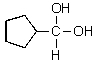
C)
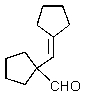
D)
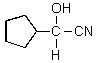
E)
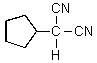

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following could not be involved in a given Wittig reaction?
A)
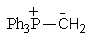
B)
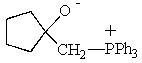
C)
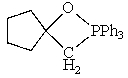
D)
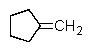
E) All of these are involved.
A)
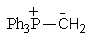
B)

C)

D)

E) All of these are involved.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following represents an oxaphosphetane intermediate formed during a Wittig reaction?
A)
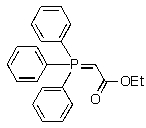
B)
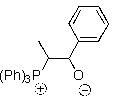
C)
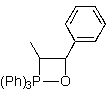
D)
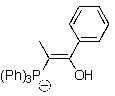
E)
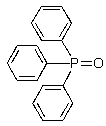
A)

B)

C)
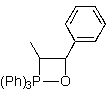
D)

E)


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
7
What would be the major product of the following reaction? 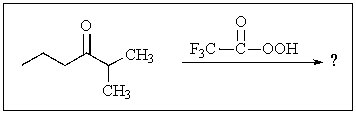
A)
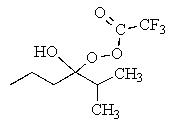
B)
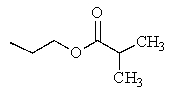
C)
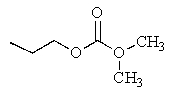
D)
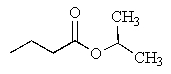
E)
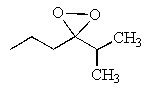

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
8
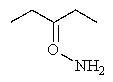
What product would you expect from the following reaction? 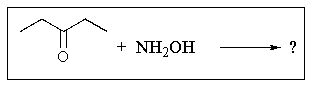
A)
B)
C)
D)
E)
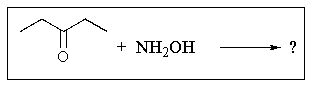
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is a typical 13C-NMR shift value for a carbonyl carbon?
A) 208 ppm
B) 29.3 ppm
C) 9.8 ppm
D) 45.2 ppm
E) 1700 cm-1
A) 208 ppm
B) 29.3 ppm
C) 9.8 ppm
D) 45.2 ppm
E) 1700 cm-1

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
10
Indicate which side,if any,would be favored in the reaction shown below. 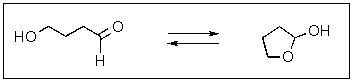
A) To the right
B) To the left
C) Equally to both sides
D) This reaction cannot occur
E) A different product would be favored.

A) To the right
B) To the left
C) Equally to both sides
D) This reaction cannot occur
E) A different product would be favored.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
11
Indicate to which side,if any,the following equilibrium lies: 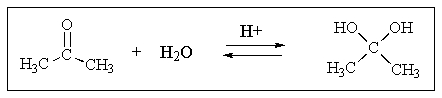
A) To the right
B) To the left
C) Equally to right and left
D) Reaction cannot occur
E) A different product is formed

A) To the right
B) To the left
C) Equally to right and left
D) Reaction cannot occur
E) A different product is formed

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
12
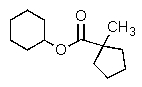
What is the major organic product of the reaction below? 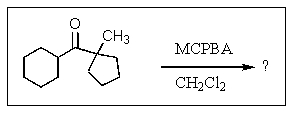
A)
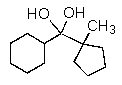
B)
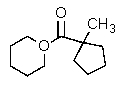
C)
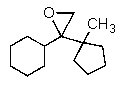
D)
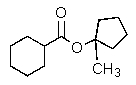
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
13
Predict the major organic product of the following reaction sequence. 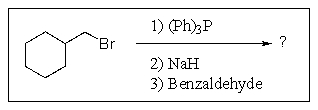
A)
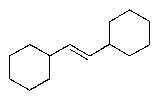
B)
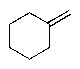
C)
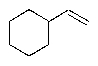
D)
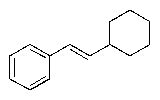
E)
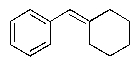

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
14
Suppose that a special catalyst was discovered that would allow an equilibrium to occur between the two molecules shown below.To which side,if any,would you expect the equilibrium to lie? 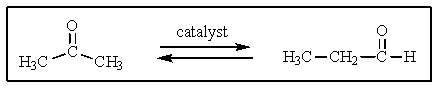
A) To the right
B) To the left
C) Equally to the right and left
D) There is no way to predict this.
E) These molecules are not isomers.

A) To the right
B) To the left
C) Equally to the right and left
D) There is no way to predict this.
E) These molecules are not isomers.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
15
What would be the best name of the following compound? 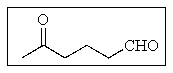
A) 6-oxo-2-hexanone
B) 5-oxohexanal
C) Hexan-5-one-1-al
D) 1,5-dioxohexane
E) 2-oxohexanal

A) 6-oxo-2-hexanone
B) 5-oxohexanal
C) Hexan-5-one-1-al
D) 1,5-dioxohexane
E) 2-oxohexanal

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
16
What product would you expect from the following reaction? (Hint: In what form does the reactant actually exist?) 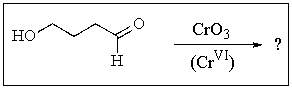
A)
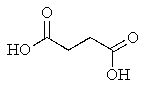
B)
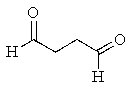
C)
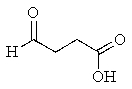
D)
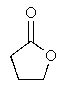
E)


A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
17
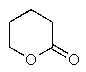
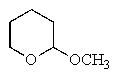
Which of the following would react with Ag+ under basic conditions?
A)
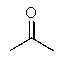
B)
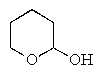
C) CH3CH2CO2CH2CH3
D)
E)
A)

B)

C) CH3CH2CO2CH2CH3
D)

E)


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
18
To which side (if any)would the following equilibrium lie? 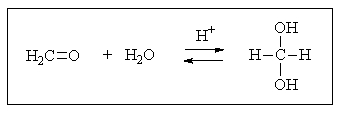
A) To left
B) To right
C) Equally to the right and left
D) Reaction cannot occur at all
E) Equilibrium favors a different product.

A) To left
B) To right
C) Equally to the right and left
D) Reaction cannot occur at all
E) Equilibrium favors a different product.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
19
Why is the reaction of the type shown below usually done? 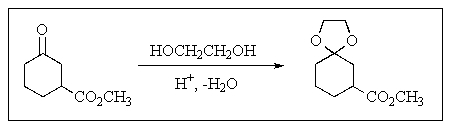
A) To make the hydrogens more acidic
B) To protect a ketone or aldehyde carbonyl
C) To make the molecule more reactive
D) To make an aldehyde or ketone less water soluble
E) To increase the oxygen content

A) To make the hydrogens more acidic
B) To protect a ketone or aldehyde carbonyl
C) To make the molecule more reactive
D) To make an aldehyde or ketone less water soluble
E) To increase the oxygen content

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
20
What test is used to detect the presence of an aldehyde?
A) Tollen's
B) Lucas
C) Fehling
D) A and B
E) A and C
A) Tollen's
B) Lucas
C) Fehling
D) A and B
E) A and C

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
21
At which atom of the following structure will a nucleophile attack? 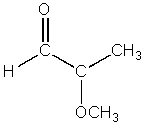
A) At the carbonyl oxygen
B) At the carbonyl carbon
C) At the methyl carbon
D) At the ether oxygen
E) At the aldehyde hydrogen

A) At the carbonyl oxygen
B) At the carbonyl carbon
C) At the methyl carbon
D) At the ether oxygen
E) At the aldehyde hydrogen

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
22
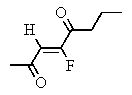
Which of the following structures represents: (Z)-3-fluoro-3-octen-2,5-dione?
A)
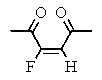
B)
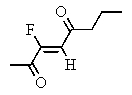
C)
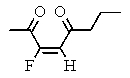
D)
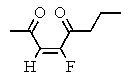
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
23
Predict the product of the following reaction: 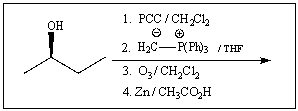
A)
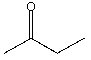
B)
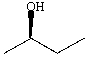
C)
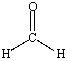
D) CH3OH
E) Both A and C

A)

B)

C)

D) CH3OH
E) Both A and C

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
24
Predict the product from the following reaction sequence. 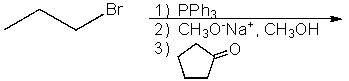
A)

B)
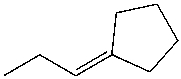
C)

D)
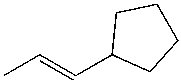
E)
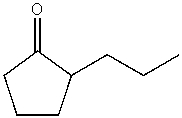

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
25
Old bottles of benzaldehyde (a liquid that smells like cherries)are often observed to have crystals on the bottom; what is the identity of this solid?
A)
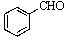
B)
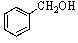
C)
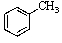
D)
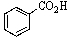
E)
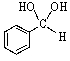
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
26
What is the name of the reaction by which aldehydes and ketones are converted directly to alkenes?
A) Friedel-Crafts
B) Williamson
C) Wittig
D) Hofmann
E) Fischer
A) Friedel-Crafts
B) Williamson
C) Wittig
D) Hofmann
E) Fischer

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
27
The IUPAC name for piperitone is 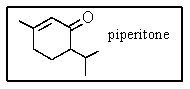
A) 6-isopropyl-3-methyl-2-cyclohexenone.
B) 2-isopropyl-5-methyl-5-cyclohexenone.
C) 6-isopropyl-3-methylcyclohexanone.
D) 1-methyl-4-isopropylcyclohexen-3-one.
E) 6-isopropyl-3-methyl-3-cyclohexenone.

A) 6-isopropyl-3-methyl-2-cyclohexenone.
B) 2-isopropyl-5-methyl-5-cyclohexenone.
C) 6-isopropyl-3-methylcyclohexanone.
D) 1-methyl-4-isopropylcyclohexen-3-one.
E) 6-isopropyl-3-methyl-3-cyclohexenone.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
28
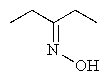
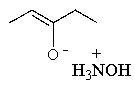
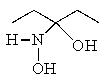
Predict the product of the following reaction: 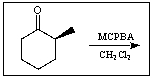
A)
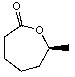
B)
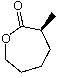
C)
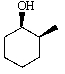
D)
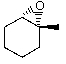
E)
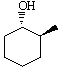

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
29
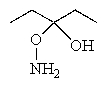
What reactants would be required to prepare the oxime shown below? 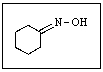
A)
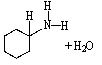
B)
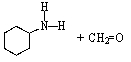
C)
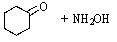
D)
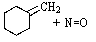
E) None of these would produce the desired product.

A)

B)
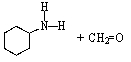
C)
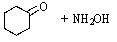
D)
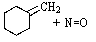
E) None of these would produce the desired product.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
30
Given the geometry of nitrogen (which is not represented properly here),the oxime shown below could exist as how many isomers? 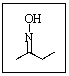
A) Only one isomer is possible.
B) Two enantiomers are possible.
C) Two diastereomers are possible.
D) Three stereoisomers are possible.
E) There are no stereoisomers of this compound possible.

A) Only one isomer is possible.
B) Two enantiomers are possible.
C) Two diastereomers are possible.
D) Three stereoisomers are possible.
E) There are no stereoisomers of this compound possible.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
31
Which is the structure of 3,7-dimethyl-2,6-octadienal?
A)
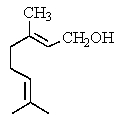
B)
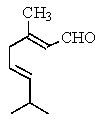
C)
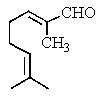
D)
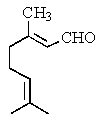
E)
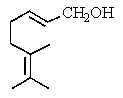
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
32
Predict the product from the following reaction. 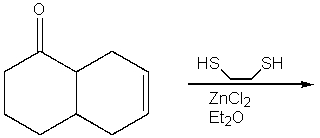
A)
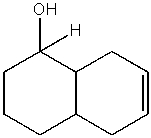
B)
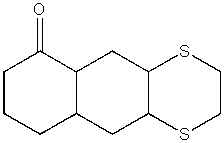
C)
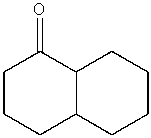
D)
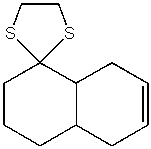
E)
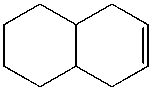

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck


