Deck 16: Electrophilic Attack on Derivatives of Benzene: Substituents Control Regioselectivity
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/30
Play
Full screen (f)
Deck 16: Electrophilic Attack on Derivatives of Benzene: Substituents Control Regioselectivity
1
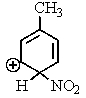
Which of the following resonance structures is the most stable?
A)
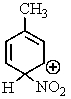
B)
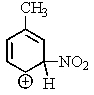
C)
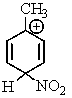
D)
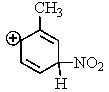
E)
A)

B)

C)

D)

E)


2
Which of the following sequences of reactants would you expect to convert benzene to the substituted aromatic shown below? 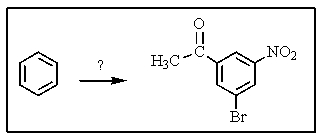
A)
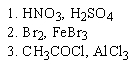
B)
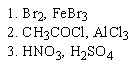
C)
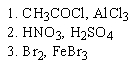
D) both A and B
E) both B and C

A)
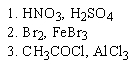
B)
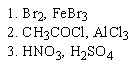
C)
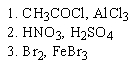
D) both A and B
E) both B and C
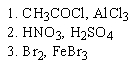
3
Predict the major organic product of the following reaction. 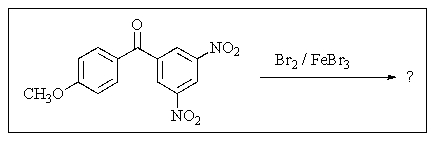
A)
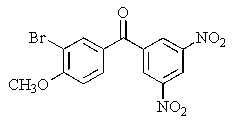
B)
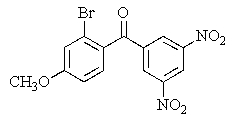
C)
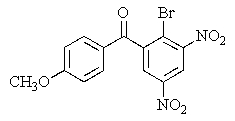
D)
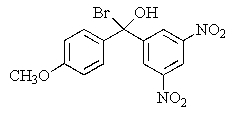
E)
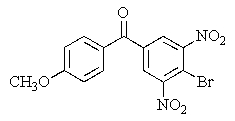

A)

B)

C)

D)

E)


4
What major product(s)would you expect from the following reaction? 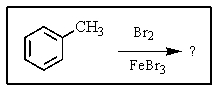
A)
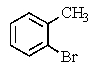
B)
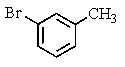
C)
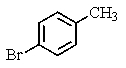
D)
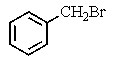
E) both A and C

A)

B)

C)

D)

E) both A and C

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
5
What major product would you expect from the following reaction? 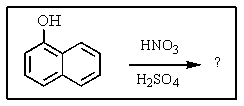
A)
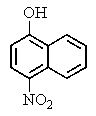
B)
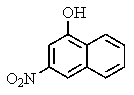
C)
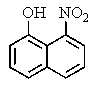
D)
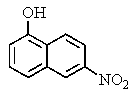
E)
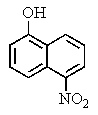

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
6
The explosive TNT is an isomer of trinitrotoluene.Given what you know about electrophilic aromatic substitution,which isomer is it most likely to be?
A)
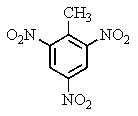
B)
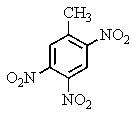
C)
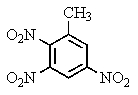
D)
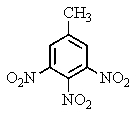
E)
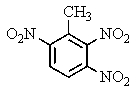
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
7
Rank the following aromatics in order of decreasing reactivity toward electrophilic aromatic substitution (most reactive > least reactive). 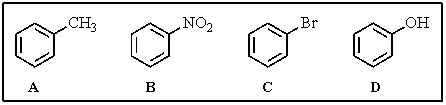
A) A > C > D > B
B) D > C > A > B
C) B > C > A > D
D) D > A > C > B
E) C > A > D > B

A) A > C > D > B
B) D > C > A > B
C) B > C > A > D
D) D > A > C > B
E) C > A > D > B

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
8
What significant product(s)would not be formed in the following reaction? 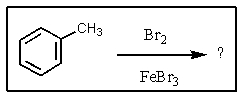
A)
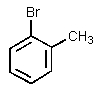
B)
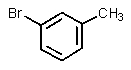
C)
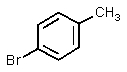
D) Neither A nor B would be formed.
E) Neither A nor C would be formed.

A)

B)

C)

D) Neither A nor B would be formed.
E) Neither A nor C would be formed.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
9
In which electrophilic aromatic substitution reaction can unintended poly-substitution (i.e.,disubstitution,trisubstitution,etc.)be a problem?
A) Nitration
B) Friedel-Crafts acylation
C) Bromination
D) Friedel-Crafts alkylation
E) Chlorination
A) Nitration
B) Friedel-Crafts acylation
C) Bromination
D) Friedel-Crafts alkylation
E) Chlorination

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
10
What major product(s)would you expect from the reaction shown? 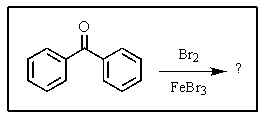
A)
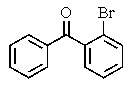
B)
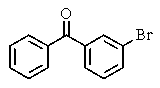
C)
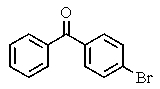
D)
E) both A and C

A)

B)

C)

D)

E) both A and C

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
11
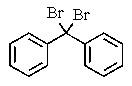
What would be the best way to prepare the ketone shown below? 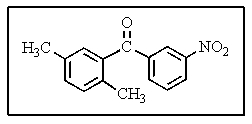
A)
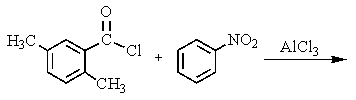
B)
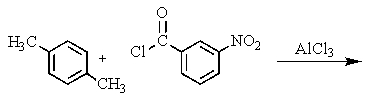
C)
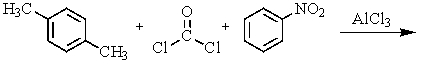
D) Any of these would work.
E) None of these would work.

A)

B)

C)
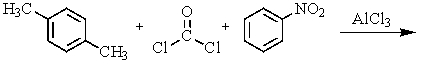
D) Any of these would work.
E) None of these would work.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
12
One of the interesting aspects of azulenes is that they are soluble in strong aqueous acids because of a reaction with H+.Which of the structures below would represent the most stable form of protonated azulene? 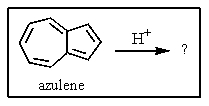
A)
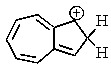
B)
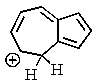
C)
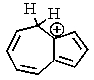
D)
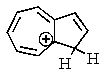
E)
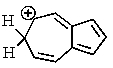

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
13
What product(s)would you expect from the following reaction? 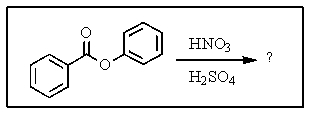
A)
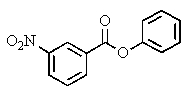
B)
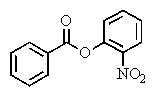
C)
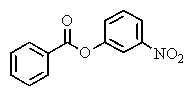
D)
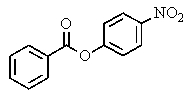
E) both B and D

A)

B)

C)

D)

E) both B and D

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
14
Carefully consider the effect of the nitro groups on the stability of the intermediates involved as you decide which product(s)the reaction shown provides. 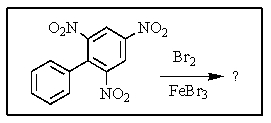
A)
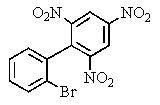
B)
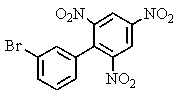
C)
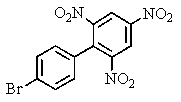
D) both A and C
E) None of these would be formed.

A)

B)

C)

D) both A and C
E) None of these would be formed.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
15
What major product(s)would you expect from the following reaction? 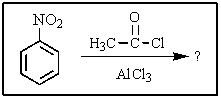
A)
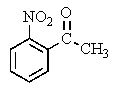
B)
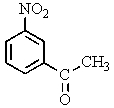
C)
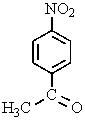
D) both A and C
E) No reaction occurs.

A)

B)

C)

D) both A and C
E) No reaction occurs.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
16
What major product(s)would you expect from the following reaction? 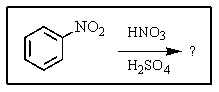
A)
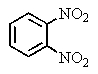
B)
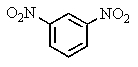
C)
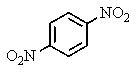
D)
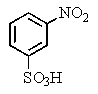
E) both A and C

A)

B)

C)

D)

E) both A and C

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements is not true of the process shown below? 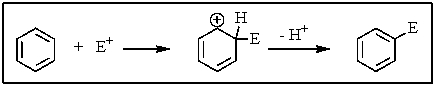
A) Benzene is less reactive with E+ than other alkenes are.
B) The overall process is a substitution reaction.
C) There are three resonance structures of the intermediate possible.
D) The first step is rate determining.
E) None of the above.All these statements are true.

A) Benzene is less reactive with E+ than other alkenes are.
B) The overall process is a substitution reaction.
C) There are three resonance structures of the intermediate possible.
D) The first step is rate determining.
E) None of the above.All these statements are true.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
18
Rank the following in order of decreasing reactivity toward nitration (more reactive > less reactive). 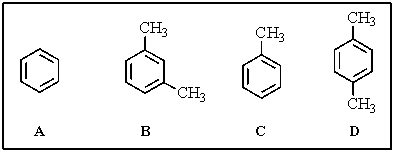
A) D > C > B > A
B) B > D > C > A
C) D > B > C > A
D) C > D > B > A
E) A > C > D > B

A) D > C > B > A
B) B > D > C > A
C) D > B > C > A
D) C > D > B > A
E) A > C > D > B

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
19
What reagent(s)would be required to accomplish the following reaction? 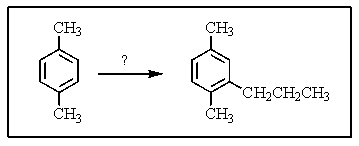
A) CH3CH2C(=O)Cl,AlCl3
B) CH3CH2CH2Cl,AlCl3
C) Reagents in A,followed by HCl,Zn(Hg),
D) Reagents in B followed by zinc and HCl
E) CH3CH=CH2,H2SO4

A) CH3CH2C(=O)Cl,AlCl3
B) CH3CH2CH2Cl,AlCl3
C) Reagents in A,followed by HCl,Zn(Hg),
D) Reagents in B followed by zinc and HCl
E) CH3CH=CH2,H2SO4

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
20
What would be the proper name of the molecule below? 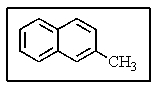
A) benzotoluene
B) 3-methylnapthalene
C) 3-methyldibenzene
D) 2-methylnapthalene
E) 1-methylnapthalene

A) benzotoluene
B) 3-methylnapthalene
C) 3-methyldibenzene
D) 2-methylnapthalene
E) 1-methylnapthalene

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
21
Choose the appropriate reagents necessary to achieve this reaction: 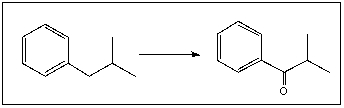
A)
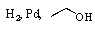
B) Zn(Hg).HCl,
C) Fe,HCl
D) CrO3,H+,H2O
E) None of these are appropriate reagents.

A)
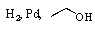
B) Zn(Hg).HCl,
C) Fe,HCl
D) CrO3,H+,H2O
E) None of these are appropriate reagents.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
22
Predict the product of the following reaction: 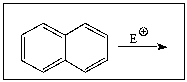
A)
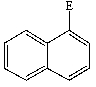
B)
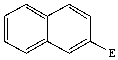
C)
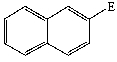
D)
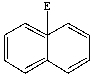
E) None of these are products.

A)

B)

C)

D)

E) None of these are products.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
23
How many lines would be seen in the 13C NMR spectrum of the product from the following reaction? 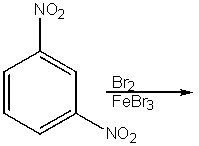
A) 2
B) 3
C) 4
D) 5
E) 6

A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following aromatics could you expect to make from benzene in the highest yield?
A)
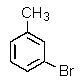
B)
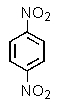
C)
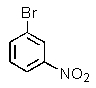
D)
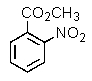
E)
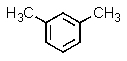
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
25
Considering the intermediate formed upon addition of an electrophile to this aromatic ring,which of the following is not a correct resonance structure: 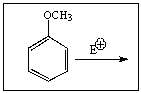
A)
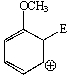
B)
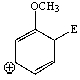
C)
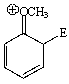
D)
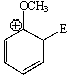
E) All of these are correct.

A)

B)

C)

D)

E) All of these are correct.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
26
What would be the major product from the following reaction? 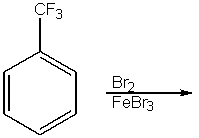
A)
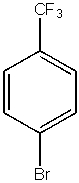
B)
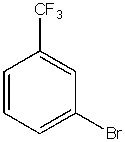
C)
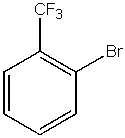
D)
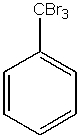
E) both A and C

A)

B)

C)

D)

E) both A and C

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
27
What would be the major product from the following reaction sequence? 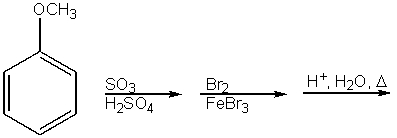
A)
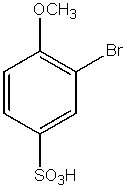
B)
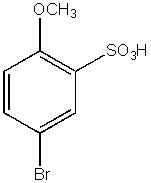
C)
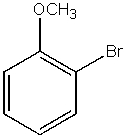
D)
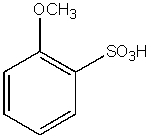
E)
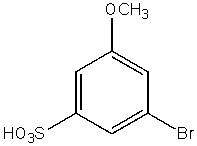

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
28
Predict the major product of the following reaction: 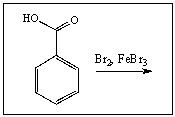
A)
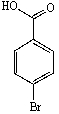
B)
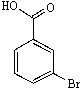
C)
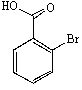
D)
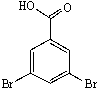
E) None of these are products.

A)

B)

C)

D)

E) None of these are products.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following are not electron-withdrawing groups?
A) Carbonyl
B) Cyano
C) Alkyl
D) Sulfonyl
E) Nitro
A) Carbonyl
B) Cyano
C) Alkyl
D) Sulfonyl
E) Nitro

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
30
Rank the following in order of increasing reactivity toward electrophilic substitution: 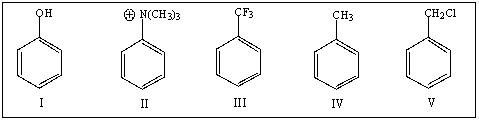
A) I,IV,V,II,III
B) II,III,V,IV,I
C) III,V,IV,I,II
D) II,V,IV,III,I
E) V,IV,II,III,I

A) I,IV,V,II,III
B) II,III,V,IV,I
C) III,V,IV,I,II
D) II,V,IV,III,I
E) V,IV,II,III,I

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck


