Deck 10: Using Nuclear Magnetic Resonance Spectroscopy to Deduce Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/43
Play
Full screen (f)
Deck 10: Using Nuclear Magnetic Resonance Spectroscopy to Deduce Structure
1
How many different types of hydrogens are present in the following molecule? 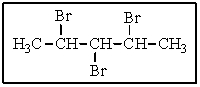
A) three
B) four
C) five
D) six
E) seven

A) three
B) four
C) five
D) six
E) seven
three
2
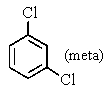
A bottle in a chemical stockroom was labeled simply "dichlorobenzene" without specifying which isomer was present.Capillary GC showed that it was a single pure compound,and the proton decoupled carbon NMR spectrum showed three peaks (not including solvent).You conclude that the bottle contained
A)
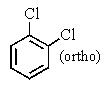
B)
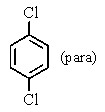
C)
D) any of the dichlorobenzene isomers
E) none of the dichlorobenzene isomers
A)

B)

C)

D) any of the dichlorobenzene isomers
E) none of the dichlorobenzene isomers

3
The most downfield proton NMR signal (i.e.,signal most to the left in the spectrum)in the following molecule would be 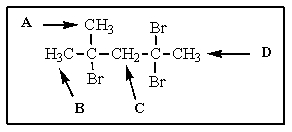
A) A
B) B
C) C
D) D
E) there is no way to tell

A) A
B) B
C) C
D) D
E) there is no way to tell
C
4
How many different signals will be present in the proton NMR for ethylpropanoate? (Do not count TMS as one of the signals!) 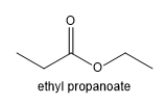
A) 2
B) 3
C) 4
D) 5
E) 6

A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
5
Heating the 4-methylbenzenesulfonate ester of the isomer shown below in 2,2,2-trifluoroethanol (a highly ionizing solvent of low nucleophilicity)leads two products with the molecular formula C10H18.The major product displays 9 different signals in its 13C NMR spectrum.Two of them occur at relatively low field,about = 120 and 145 ppm,respectively.The 1H NMR spectrum exhibits a multiplet near = 5 ppm (1 H); all other signals are upfield of = 3 ppm.Identify the compound. 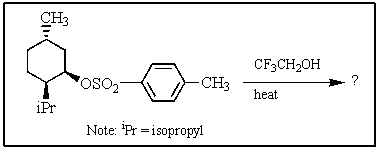
A)
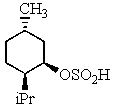
B)
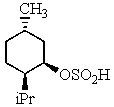
C)
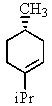
D)
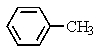
E)
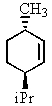

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
6
The type of electromagnetic energy required to cause nuclear magnetic resonance is
A) infrared.
B) radio.
C) ultraviolet.
D) visible.
E) microwave.
A) infrared.
B) radio.
C) ultraviolet.
D) visible.
E) microwave.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
7
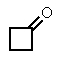
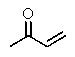
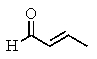
The molecular formulas and 13C NMR data (in ppm)are given below.The splitting pattern of each signal,taken from the non-decoupled spectrum is given in parentheses.Deduce the correct structure: 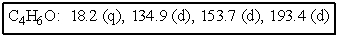
A)
B)
C)
D)
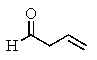
E)
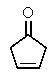
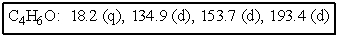
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
8
The molecular formulas and 13C NMR data (in ppm)are given below.The splitting pattern of each signal,taken from the non-decoupled spectrum is given in parentheses.Deduce the correct structure: 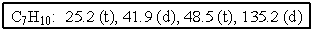
A)
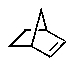
B)
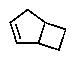
C)
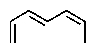
D)
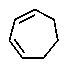
E)
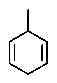
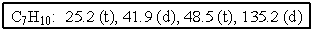
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
9
In nuclear magnetic resonance,stronger magnetic fields
A) give a higher sensitivity spectrum than do lower magnetic fields.
B) give different chemical shifts (in ppm)than would weaker magnets.
C) give better separation between peaks in the spectrum (in Hz)than would weaker magnets.
D) give different coupling constants than would be observed with weaker magnetic fields.
E) Both A and C are true.
A) give a higher sensitivity spectrum than do lower magnetic fields.
B) give different chemical shifts (in ppm)than would weaker magnets.
C) give better separation between peaks in the spectrum (in Hz)than would weaker magnets.
D) give different coupling constants than would be observed with weaker magnetic fields.
E) Both A and C are true.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
10
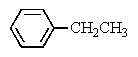
Which compound most likely exhibits the following proton NMR? 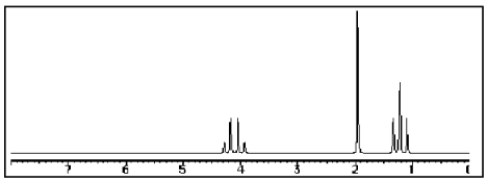
A)
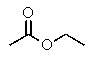
B)
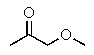
C)
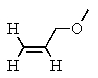
D)
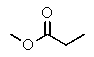
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
11
The CH2 group indicated would most likely appear as what in the proton NMR spectrum? 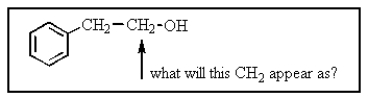
A) singlet
B) doublet
C) triplet
D) quartet
E) pentet

A) singlet
B) doublet
C) triplet
D) quartet
E) pentet

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following structures of formula C8H8Br2 would give the NMR spectrum shown below? 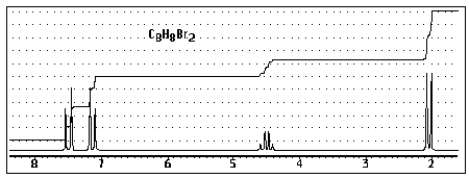
A)
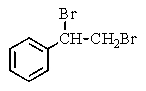
B)
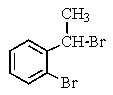
C)
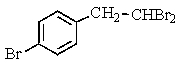
D)
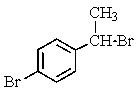
E)
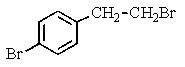

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
13
How many different types of carbon would be present in the following molecule? 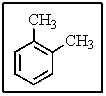
A) three
B) four
C) five
D) six
E) eight

A) three
B) four
C) five
D) six
E) eight

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following structures will give three signals (not counting TMS)in the proton decoupled 13C NMR?
A)
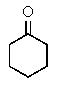
B)
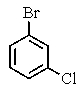
C)
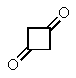
D)
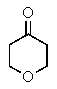
E)
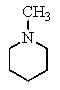
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
15
Acetone would show how many doublets in the proton-coupled 13C NMR spectrum?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
16
The natural abundance of 13C is
A) 2.1%.
B) 10%.
C) 1.5%.
D) 1.1%.
E) 16%.
A) 2.1%.
B) 10%.
C) 1.5%.
D) 1.1%.
E) 16%.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following structures C5H12O would give the NMR spectrum shown? 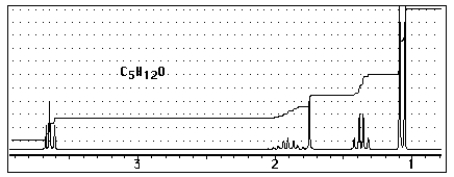
A)
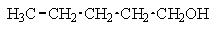
B)
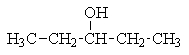
C)
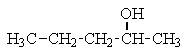
D)
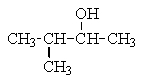
E)
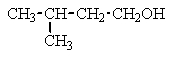

A)
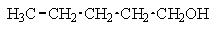
B)
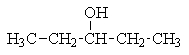
C)
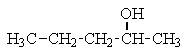
D)

E)
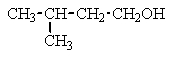

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is not true of 13C NMR?
A) The sensitivity of 13C NMR is much less than that of proton NMR.
B) By simultaneously decoupling the proton region,13C peaks appear as singlets.
C) (13C chemical shifts cover a much larger range than do 1H chemical shifts.)
D) The 13C NMR spectrum can show more peaks than there are carbons in the molecular formula.
E) The 13C NMR spectrum will show one peak for each type of carbon in a molecule.
A) The sensitivity of 13C NMR is much less than that of proton NMR.
B) By simultaneously decoupling the proton region,13C peaks appear as singlets.
C) (13C chemical shifts cover a much larger range than do 1H chemical shifts.)
D) The 13C NMR spectrum can show more peaks than there are carbons in the molecular formula.
E) The 13C NMR spectrum will show one peak for each type of carbon in a molecule.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
19
The NMR peak intensities for a first-order quartet are:
A) 1:2:2:1
B) 1:3:3:1
C) 1:4:4:1
D) 1:1:1:1
E) None of the above.
A) 1:2:2:1
B) 1:3:3:1
C) 1:4:4:1
D) 1:1:1:1
E) None of the above.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
20
A very old bottle labeled only "chlorinated benzene" was found in the stockroom.Capillary GC showed (surprisingly)that the compound was pure,and a proton-decoupled 13C NMR spectrum showed only two peaks.Which of the following compounds was in the bottle?
A)
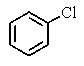
B)
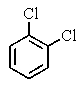
C)
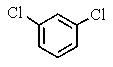
D)
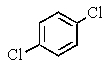
E)
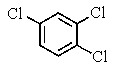
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
21
What structure of formula C3H6Br2 would give the following proton NMR spectrum? 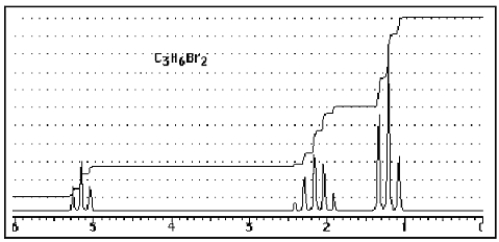
A)
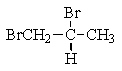
B)
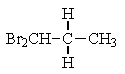
C)
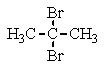
D)
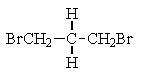
E) None of the above.

A)
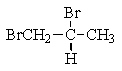
B)

C)
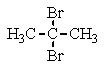
D)

E) None of the above.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is not true about the information from a proton NMR spectrum?
A) It identifies the number of different types of hydrogens in a molecule.
B) It gives the ratios of one type of hydrogen to another.
C) It gives information about what environment each hydrogen is in.
D) It tells how many neighboring hydrogens each type of hydrogen has.
E) None of the above.Proton NMR gives all these types of information.
A) It identifies the number of different types of hydrogens in a molecule.
B) It gives the ratios of one type of hydrogen to another.
C) It gives information about what environment each hydrogen is in.
D) It tells how many neighboring hydrogens each type of hydrogen has.
E) None of the above.Proton NMR gives all these types of information.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
23
How many magnetically different types of hydrogens and carbons are in the following compound? 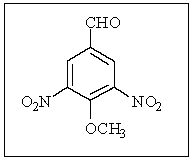
A) 6 hydrogens,8 carbons
B) 3 hydrgens,8 carbons
C) 6 hydrogens,6 carbons
D) 3 hydrogens,6 carbons
E) 3 hydrogens,3 carbons

A) 6 hydrogens,8 carbons
B) 3 hydrgens,8 carbons
C) 6 hydrogens,6 carbons
D) 3 hydrogens,6 carbons
E) 3 hydrogens,3 carbons

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
24
How many signals will be present in a decoupled 13C NMR spectrum for the molecule below? 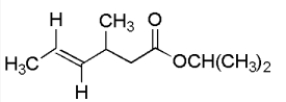
A) 7
B) 8
C) 9
D) 10
E) None of the above.

A) 7
B) 8
C) 9
D) 10
E) None of the above.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
25
For the compound below give the spin-spin splitting that would be observed for each of the protons sets in the 1H NMR spectrum. 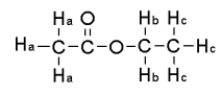
A) Ha = triplet Hb = doublet,Hc = quartet
B) Ha = singlet Hb = quartet,Hc = triplet
C) Ha = singlet Hb = pentet,Hc = quartet
D) Ha = triplet Hb = quartet,Hc = singlet
E) None of the above.
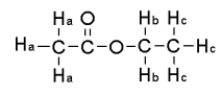
A) Ha = triplet Hb = doublet,Hc = quartet
B) Ha = singlet Hb = quartet,Hc = triplet
C) Ha = singlet Hb = pentet,Hc = quartet
D) Ha = triplet Hb = quartet,Hc = singlet
E) None of the above.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
26
A compound of the formula C10H14 gave the 1H NMR spectrum shown below and exhibited three peaks in the 13C NMR spectrum.What compound might it be? 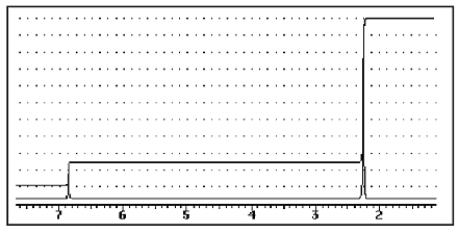
A)
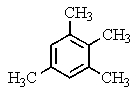
B)
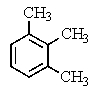
C)
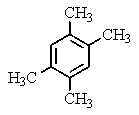
D)
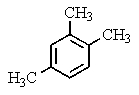
E)
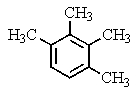

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
27
Typical hydrogen chemical shifts for aromatic protons occur in the range from
A)
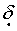 0.8-2.0.
0.8-2.0.
B)
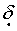 7.0-8.0.
7.0-8.0.
C)
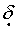 0.5-5.0.
0.5-5.0.
D)
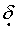 3.3-4.0.
3.3-4.0.
E) None of the above.
A)
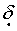 0.8-2.0.
0.8-2.0.B)
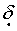 7.0-8.0.
7.0-8.0.C)
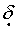 0.5-5.0.
0.5-5.0.D)
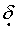 3.3-4.0.
3.3-4.0.E) None of the above.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
28
What structure would be consistent with the following NMR spectrum and data? 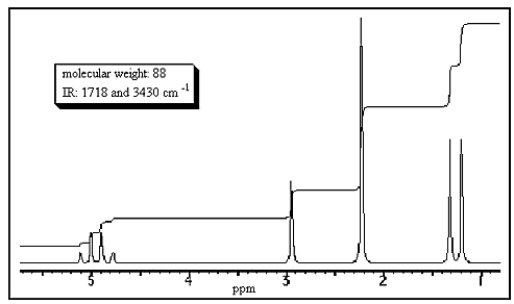
A)
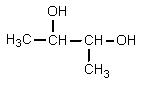
B)
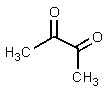
C)
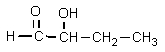
D)
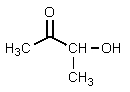
E)
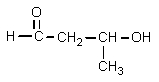

A)

B)

C)
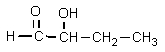
D)

E)
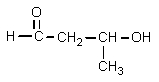

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
29
Consider the 1H-NMR of the molecule shown below.Assume that JCB = 7 Hz and JCA = 1 Hz.What coupling pattern will Hc exhibit? 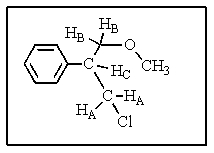
A) pentet
B) quartet
C) doublet of quartets
D) triplet of triplets
E) doublet of triplets

A) pentet
B) quartet
C) doublet of quartets
D) triplet of triplets
E) doublet of triplets

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
30
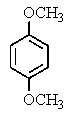
The following 1H-NMR spectrum was most likely obtained from which of the compounds listed below? 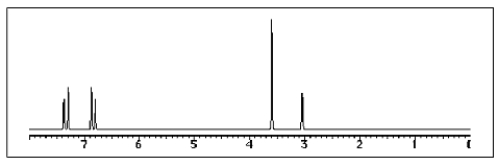
A)
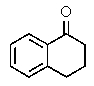
B)
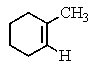
C)
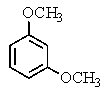
D)
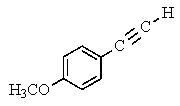
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
31
The proton NMR spectrum of a carboxylic acid,C13H18O2,commonly sold as an over-the-counter headache remedy,is shown below.Note: the CO2H proton is not shown,and the multiplicity of the peaks are noted (d = doublet,q = quartet,m = multiplet).Which structure is that of this pharmaceutical? 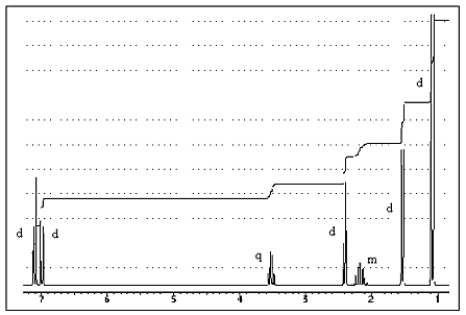
A)
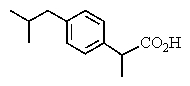
B)
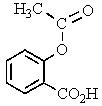
C)
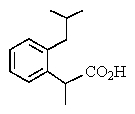
D)
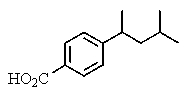
E)
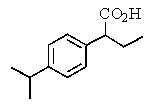

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
32
Carbon-13 NMR is
A) impossible since 13C has no magnetic moment.
B) complicated by 13C-13C splitting.
C) impossible since 13C does not occur in nature.
D) more difficult than 1H NMR.
E) None of the above.
A) impossible since 13C has no magnetic moment.
B) complicated by 13C-13C splitting.
C) impossible since 13C does not occur in nature.
D) more difficult than 1H NMR.
E) None of the above.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
33
For the compound below give the integration ratio that would be observed for each of the protons sets in the 1H NMR spectrum. 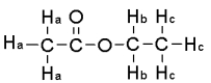
A) Ha = 3,Hb = 2,Hc = 1
B) Ha = 1 Hb = 2,Hc = 1
C) Ha = 3 Hb = 2,Hc = 3
D) Ha = 1 Hb = 1,Hc = 1
E) None of the above.

A) Ha = 3,Hb = 2,Hc = 1
B) Ha = 1 Hb = 2,Hc = 1
C) Ha = 3 Hb = 2,Hc = 3
D) Ha = 1 Hb = 1,Hc = 1
E) None of the above.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following nuclei are incapable of magnetic resonance?
A) Hydrogen (1H)
B) Carbon (13C)
C) Fluorine (19F)
D) Phosphorus (31P)
E) All are NMR active.
A) Hydrogen (1H)
B) Carbon (13C)
C) Fluorine (19F)
D) Phosphorus (31P)
E) All are NMR active.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
35
The following spectra data was most likely obtained from which compound? 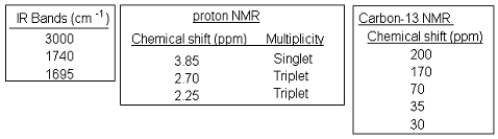
A)
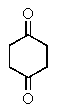
B)
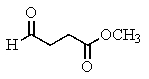
C)
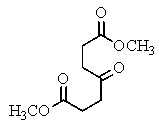
D)
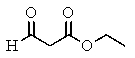
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
36
A seven-carbon compound which gives three signals in the 13C NMR spectrum could be:
A) heptane
B) 2-methylhexane
C) 3,3-dimethylpentane
D) 2,4-dimethylpentane
E) 2,2,3-trimethylbutane
A) heptane
B) 2-methylhexane
C) 3,3-dimethylpentane
D) 2,4-dimethylpentane
E) 2,2,3-trimethylbutane

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
37
A chemist is evaluating a proton NMR spectrum taken on a 300 MHz NMR spectrometer.She wishes to determine the chemical shift in ppm of a singlet that appears at 1140 Hz from TMS.What is the chemical shift in ppm?
A) 0.26 ppm
B) 3.8 ppm
C) 7.6 ppm
D) 300 ppm
E) None of the above.
A) 0.26 ppm
B) 3.8 ppm
C) 7.6 ppm
D) 300 ppm
E) None of the above.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
38
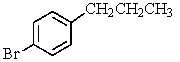
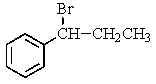
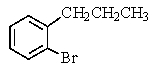
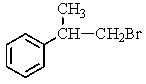
Which compound C9H11Br would give the proton NMR spectrum shown below? 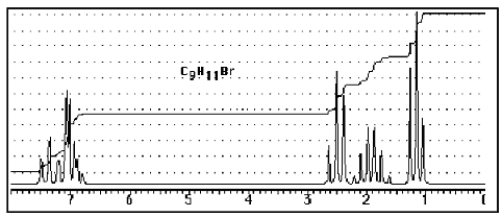
A)
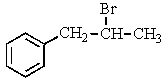
B)
C)
D)
E)

A)
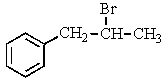
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
39
The following 1H-NMR was most likely obtained from which of the compounds listed below? 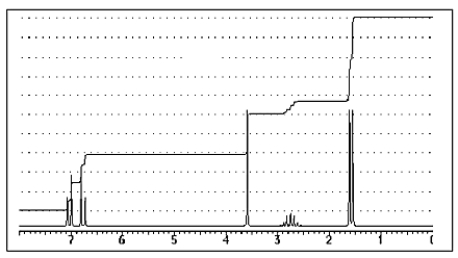
A)
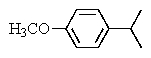
B)
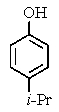
C)
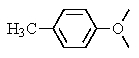
D)
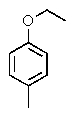
E)
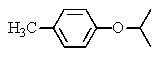

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
40
In the proton-coupled 13C-NMR spectrum of the following molecule,how many doublets would be observed? 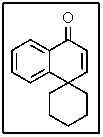
A) 2
B) 4
C) 6
D) 8
E) None of the above.

A) 2
B) 4
C) 6
D) 8
E) None of the above.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
41
Arrange each of the following labeled hydrogens in the order of increasing chemical shift. 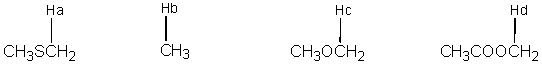
A) Ha < Hb < Hc < Hd
B) Hb < Hc < Ha < Hd
C) Hd < Hc < Ha < Hb
D) Hb < Ha < Hc < Hd
E) Hb < Ha < Hd < Hc
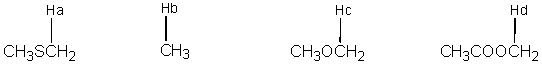
A) Ha < Hb < Hc < Hd
B) Hb < Hc < Ha < Hd
C) Hd < Hc < Ha < Hb
D) Hb < Ha < Hc < Hd
E) Hb < Ha < Hd < Hc

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
42
Describe the splitting that would be observed by Ha in the proton NMR spectrum,assuming Ha is coupled to all its neighboring protons in an equivalent manner. 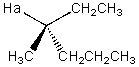
A) Ha will be split into a pentet.
B) Ha will be split into a sextet.
C) Ha will be split into a septet.
D) Ha will be split into an octet.
E) None of the above.

A) Ha will be split into a pentet.
B) Ha will be split into a sextet.
C) Ha will be split into a septet.
D) Ha will be split into an octet.
E) None of the above.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
43
Indicate how 1H NMR spectroscopy can be used to distinguish between the following sets of structures. 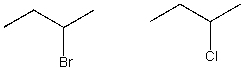
A) The spin-spin splitting for the protons on the carbon next to the halogen will be different.
B) The chemical shift of the resonance for the protons on the carbon attached to the chlorine atom will be shifted downfield relative to the protons on the carbon attached to the bromine atom.
C) The chemical shift of the resonance for the protons on the carbon attached to the chlorine atom will be shifted upfield relative to the protons on the carbon attached to the bromine atom.
D) The integration of the protons on the carbon next to the halogen will be different.
E) None of the above.
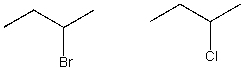
A) The spin-spin splitting for the protons on the carbon next to the halogen will be different.
B) The chemical shift of the resonance for the protons on the carbon attached to the chlorine atom will be shifted downfield relative to the protons on the carbon attached to the bromine atom.
C) The chemical shift of the resonance for the protons on the carbon attached to the chlorine atom will be shifted upfield relative to the protons on the carbon attached to the bromine atom.
D) The integration of the protons on the carbon next to the halogen will be different.
E) None of the above.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck


