Deck 17: Carbonyl Alpha-Substitution and Condensation Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/46
Play
Full screen (f)
Deck 17: Carbonyl Alpha-Substitution and Condensation Reactions
1
Which of the following is common to both tautomers and resonance forms of a compound?
A) have the same molecular formula
B) differ only in the position of electrons
C) rapidly interconvertible
D) differ in connectivity of atoms
E) all of these describe both tautomers and resonance forms
A) have the same molecular formula
B) differ only in the position of electrons
C) rapidly interconvertible
D) differ in connectivity of atoms
E) all of these describe both tautomers and resonance forms
have the same molecular formula
2
Consider the reaction sequence below to answer the following question(s). 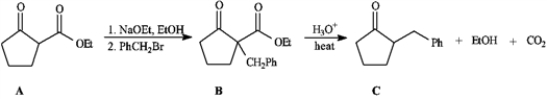
Refer to instructions. Conversion of A into B is a type of reaction termed _____.
A) an acylation
B) an enolation
C) an alkylation
D) a phenylation

Refer to instructions. Conversion of A into B is a type of reaction termed _____.
A) an acylation
B) an enolation
C) an alkylation
D) a phenylation
an alkylation
3
Refer to the compounds below to answer the following question(s). 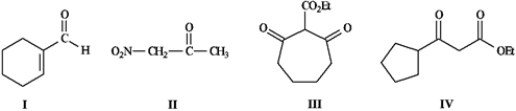
Refer to instructions. Draw the structure for the enol and enolate ions corresponding to Compound I.

Refer to instructions. Draw the structure for the enol and enolate ions corresponding to Compound I.

4
The common feature of α-substitution and condensation reactions of carbonyl groups:
A) involve two carbonyl partners
B) involve the formation of an enol or enolate ion
C) involve a nucleophile
D) produce a new carbon to carbon bond
E) all of these describe both types of reactions
A) involve two carbonyl partners
B) involve the formation of an enol or enolate ion
C) involve a nucleophile
D) produce a new carbon to carbon bond
E) all of these describe both types of reactions

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
5
Give the major organic product(s) of each reaction or sequences of reactions for the following question(s). Show all relevant stereochemistry.
Give major product(s):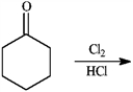
Give major product(s):


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
6
Refer to the compounds below to answer the following question(s). 
Refer to instructions. Indicate which hydrogens in Compound II are the most acidic. Explain your answer.

Refer to instructions. Indicate which hydrogens in Compound II are the most acidic. Explain your answer.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following would form an enolate ion on treatment with a base? 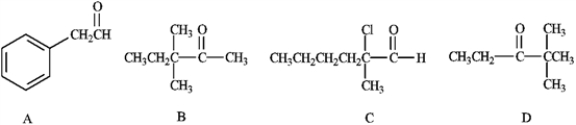
A) A
B) B
C) C
D) D
E) All of these except C
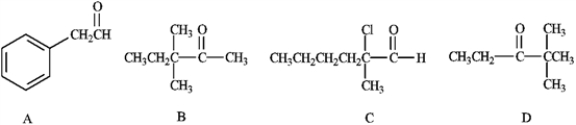
A) A
B) B
C) C
D) D
E) All of these except C

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
8
Give the major organic product(s) of each reaction or sequences of reactions for the following question(s). Show all relevant stereochemistry.
How would you prepare 3-phenylpropanoic acid using a malonic ester synthesis?
How would you prepare 3-phenylpropanoic acid using a malonic ester synthesis?

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
9
Refer to the compounds below to answer the following question(s). 
Refer to instructions. Choose the most acidic compound from Compounds I − IV. Explain your choice.

Refer to instructions. Choose the most acidic compound from Compounds I − IV. Explain your choice.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
10
An enolate ion
A) is a resonance hybrid.
B) can be protonated to from the corresponding enol.
C) can be protonated to form the keto tautomer.
D) forms under basic conditions.
E) All of these
A) is a resonance hybrid.
B) can be protonated to from the corresponding enol.
C) can be protonated to form the keto tautomer.
D) forms under basic conditions.
E) All of these

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
11
Give the major organic product(s) of each reaction or sequences of reactions for the following question(s). Show all relevant stereochemistry.
How would you prepare 5-methyl-2-hexanone using an acetoacetic ester synthesis?
How would you prepare 5-methyl-2-hexanone using an acetoacetic ester synthesis?

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
12
Give the major organic product(s) of each reaction or sequences of reactions for the following question(s). Show all relevant stereochemistry.
Give major product(s):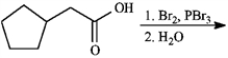
Give major product(s):


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
13
Consider the structures below to answer the following question(s). 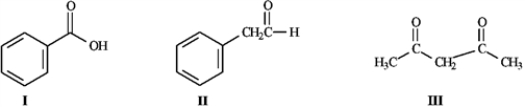
Refer to instructions. Underline the most acidic hydrogen atoms in each of the molecules.

Refer to instructions. Underline the most acidic hydrogen atoms in each of the molecules.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
14
Consider the structures below to answer the following question(s). ![Consider the structures below to answer the following question(s). Nitroethane [CH<sub>3</sub>CH<sub>2</sub>NO<sub>2</sub>, pK<sub>a</sub> = 8.6] is a much stronger acid than ethane [CH<sub>3</sub>CH<sub>3</sub>, pK<sub>a</sub> ≈ 60]. Explain.](https://storage.examlex.com/TB6688/11eab600_081c_9e9a_9625_29058f446ae3_TB6688_00_TB6688_00_TB6688_00.jpg)
Nitroethane [CH3CH2NO2, pKa = 8.6] is a much stronger acid than ethane [CH3CH3, pKa ≈ 60]. Explain.
![Consider the structures below to answer the following question(s). Nitroethane [CH<sub>3</sub>CH<sub>2</sub>NO<sub>2</sub>, pK<sub>a</sub> = 8.6] is a much stronger acid than ethane [CH<sub>3</sub>CH<sub>3</sub>, pK<sub>a</sub> ≈ 60]. Explain.](https://storage.examlex.com/TB6688/11eab600_081c_9e9a_9625_29058f446ae3_TB6688_00_TB6688_00_TB6688_00.jpg)
Nitroethane [CH3CH2NO2, pKa = 8.6] is a much stronger acid than ethane [CH3CH3, pKa ≈ 60]. Explain.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following would form an enol on treatment with an acid?
A) 4-chlorobenzaldehye
B) 2,2,-dimethylbutanal
C) 2,2,4,4-tetrachlorohexan-3-one
D) none of these
A) 4-chlorobenzaldehye
B) 2,2,-dimethylbutanal
C) 2,2,4,4-tetrachlorohexan-3-one
D) none of these

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
16
Give the major organic product(s) of each reaction or sequences of reactions for the following question(s). Show all relevant stereochemistry.
Refer to instructions.
a)Give major product(s):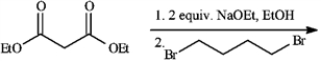 b)Why are two equivalents of Na+ OEt− required?
b)Why are two equivalents of Na+ OEt− required?
Refer to instructions.
a)Give major product(s):
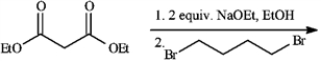 b)Why are two equivalents of Na+ OEt− required?
b)Why are two equivalents of Na+ OEt− required?
Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
17
Consider the structures below to answer the following question(s). 
Refer to instructions. Rank the molecules above in order of increasing acidity (least acidic to most acidic).
A) III, II, I
B) II, III, I
C) I, II, III
D) II, I, III

Refer to instructions. Rank the molecules above in order of increasing acidity (least acidic to most acidic).
A) III, II, I
B) II, III, I
C) I, II, III
D) II, I, III

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
18
Refer to the compounds below to answer the following question(s). 
Refer to instructions. Underline all the acidic hydrogen atoms in Compounds I through IV.

Refer to instructions. Underline all the acidic hydrogen atoms in Compounds I through IV.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
19
Consider the reaction sequence below to answer the following question(s). 
Refer to instructions. The starting material A in this reaction sequence is called a(n) _____.
A) β-keto ester
B) α-carboethoxy ketone
C) malonic ester
D) acetoacetic ester

Refer to instructions. The starting material A in this reaction sequence is called a(n) _____.
A) β-keto ester
B) α-carboethoxy ketone
C) malonic ester
D) acetoacetic ester

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
20
Consider the reaction sequence below to answer the following question(s). 
Refer to instructions. Conversion of B into C involves hydrolysis of the ester followed by decarboxylation. On the structures provided below, show the electron flow for the decarboxylation step.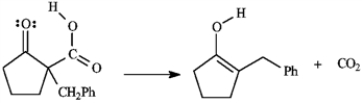

Refer to instructions. Conversion of B into C involves hydrolysis of the ester followed by decarboxylation. On the structures provided below, show the electron flow for the decarboxylation step.


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
21
Draw the structures of the precursors to the Michael reaction products shown in the question(s) below. Label the Michael donor and the Michael acceptor in each case and formulate the reaction.
Draw and label: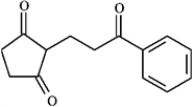
Draw and label:


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
22
Draw the structures of the precursors to the Michael reaction products shown in the question(s) below. Label the Michael donor and the Michael acceptor in each case and formulate the reaction.
Draw and label: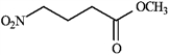
Draw and label:


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
23
Draw the structure of the product you would expect to obtain by Claisen condensation of the esters shown in the question(s) below. If an ester does not undergo Claisen condensation, explain why it does not.
Draw and explain: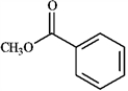
Draw and explain:


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
24
Each of the following compounds in the following question(s) can be prepared by a mixed aldol condensation reaction.a)
Give the structures of the aldehyde and/or ketone precursors for each aldol condensation product and formulate the reaction.b)
Give the structure of the intermediate aldol product.
Refer to instructions. Use the following compound: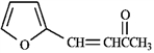
Give the structures of the aldehyde and/or ketone precursors for each aldol condensation product and formulate the reaction.b)
Give the structure of the intermediate aldol product.
Refer to instructions. Use the following compound:


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
25
Draw the structure of the aldol self-condensation product of each compound for the following question(s). If a compound does not undergo aldol self-condensation, explain why it does not.
Draw and explain: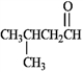
Draw and explain:
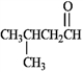

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the reaction below to answer the following question(s). 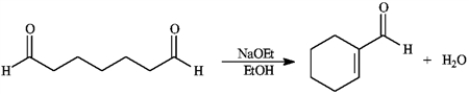
Refer to instructions. Write the complete stepwise mechanism for the reaction above. Show all intermediate structures and all electron flow with arrows.

Refer to instructions. Write the complete stepwise mechanism for the reaction above. Show all intermediate structures and all electron flow with arrows.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
27
Each of the following compounds in the following question(s) can be prepared by a mixed aldol condensation reaction.a)
Give the structures of the aldehyde and/or ketone precursors for each aldol condensation product and formulate the reaction.b)
Give the structure of the intermediate aldol product.
Refer to instructions. Use the following compound: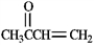
Give the structures of the aldehyde and/or ketone precursors for each aldol condensation product and formulate the reaction.b)
Give the structure of the intermediate aldol product.
Refer to instructions. Use the following compound:
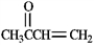

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
28
Draw the structure of the aldol self-condensation product of each compound for the following question(s). If a compound does not undergo aldol self-condensation, explain why it does not.
Draw and explain: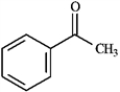
Draw and explain:


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
29
Consider the reaction below to answer the following question(s). 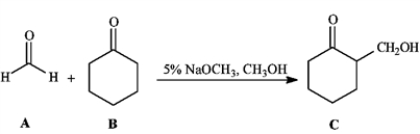
Refer to instructions. Draw the structure of the enolate ion that is generated during the course of this reaction.
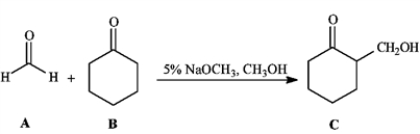
Refer to instructions. Draw the structure of the enolate ion that is generated during the course of this reaction.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the reaction below to answer the following question(s). 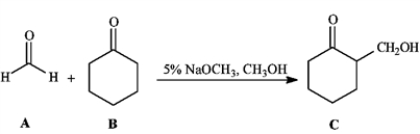
Refer to instructions. Which carbonyl compound functions as the electrophile in this reaction?
A)A
B)B
C)C
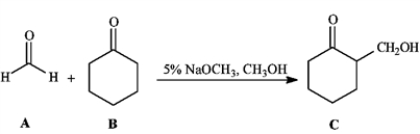
Refer to instructions. Which carbonyl compound functions as the electrophile in this reaction?
A)A
B)B
C)C

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
31
Draw the structure of the aldol self-condensation product of each compound for the following question(s). If a compound does not undergo aldol self-condensation, explain why it does not.
Draw and explain: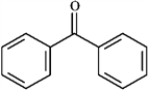
Draw and explain:


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
32
Draw the structures of the precursors to the Michael reaction products shown in the question(s) below. Label the Michael donor and the Michael acceptor in each case and formulate the reaction.
Draw and label: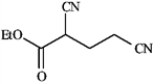
Draw and label:


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
33
Consider the reaction below to answer the following question(s). 
Refer to instructions. The product of this reaction is:
A) a β, γ-unsaturated aldehyde
B) an α, β-unsaturated ketone
C) an α, β-unsaturated aldehyde
D) an enol

Refer to instructions. The product of this reaction is:
A) a β, γ-unsaturated aldehyde
B) an α, β-unsaturated ketone
C) an α, β-unsaturated aldehyde
D) an enol

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the reaction below to answer the following question(s). 
Refer to instructions. This reaction is an example of:
A) an intramolecular Claisen condensation
B) an intramolecular aldol condensation
C) a Robinson annulation
D) a Michael reaction

Refer to instructions. This reaction is an example of:
A) an intramolecular Claisen condensation
B) an intramolecular aldol condensation
C) a Robinson annulation
D) a Michael reaction

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
35
Consider the reaction below to answer the following question(s). 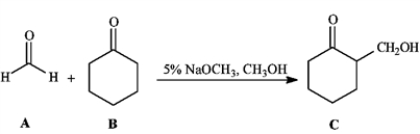
Refer to instructions. This reaction is an example of:
A) a mixed Claisen condensation.
B) a Dieckman condensation.
C) a Michael reaction.
D) a mixed aldol reaction.
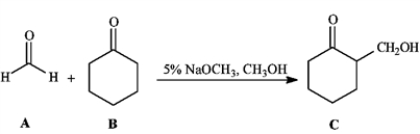
Refer to instructions. This reaction is an example of:
A) a mixed Claisen condensation.
B) a Dieckman condensation.
C) a Michael reaction.
D) a mixed aldol reaction.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
36
Draw the structure of the product you would expect to obtain by Claisen condensation of the esters shown in the question(s) below. If an ester does not undergo Claisen condensation, explain why it does not.
Draw and explain: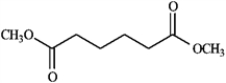
Draw and explain:


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following does not possess an enol form? Explain your choice. 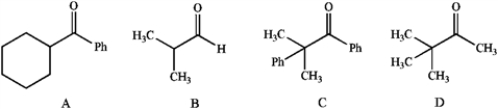


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
38
The Stork enamine reaction is a variation on the Michael reaction that utilizes an enamine nucleophile. Use this information to answer the following question. 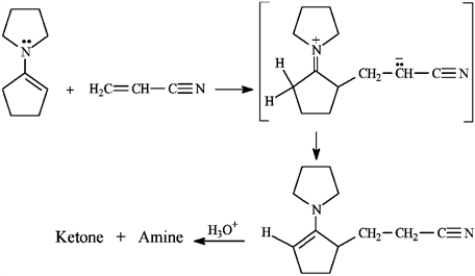
Refer to instructions. Draw the structures of the ketone and amine products of this reaction.

Refer to instructions. Draw the structures of the ketone and amine products of this reaction.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
39
Rank the following hydrogens in terms of decreasing acidity (most acidic > least acidic): 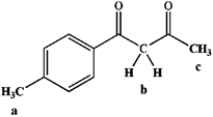


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
40
Each of the following compounds in the following question(s) can be prepared by a mixed aldol condensation reaction.a)
Give the structures of the aldehyde and/or ketone precursors for each aldol condensation product and formulate the reaction.b)
Give the structure of the intermediate aldol product.
Refer to instructions. Use the following compound: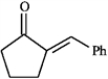
Give the structures of the aldehyde and/or ketone precursors for each aldol condensation product and formulate the reaction.b)
Give the structure of the intermediate aldol product.
Refer to instructions. Use the following compound:


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
41
How would you prepare the following compound using an alkylation reaction? 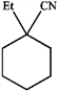


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
42
Identify products a and
b.
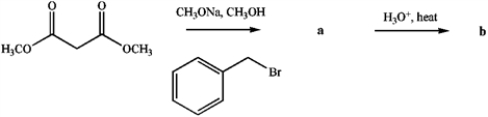
b.


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
43
Predict the major aldol product of the following reaction. 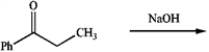
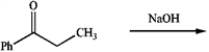

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
44
Identify the intermediate imine that results from the following reaction. 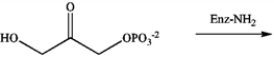


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
45
When treated with base and heat, the following diketone undergoes an intramolecular aldol reaction followed by dehydration to produce cis-jasmone, a perfume component. Draw the structure of the product. 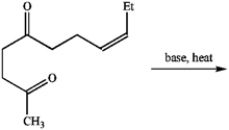


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
46
Draw the structure of the diastereomer that is formed preferentially in the following reaction: 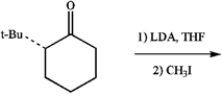


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck


