Deck 18: Amines and Heterocycles
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/36
Play
Full screen (f)
Deck 18: Amines and Heterocycles
1
Give the major organic product(s) of each of the following reaction or sequence of reactions. Show all relevant stereochemistry.
Give major product(s):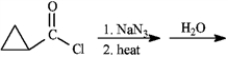
Give major product(s):
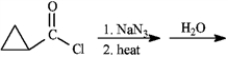
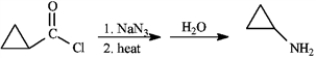
2
Draw structures corresponding to each of the IUPAC names given in the following question(s).
Draw:
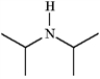
diisopropylamine
Draw:
diisopropylamine
3
In the following series of compounds, which is the most basic and the least basic? Explain your choices. 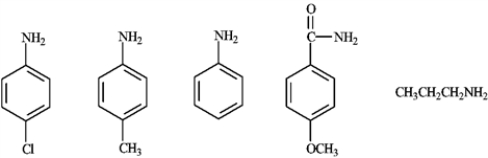

CH3CH2CH2NH2
Strongest base because it is the only aliphatic amine.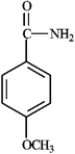 Weakest based because it is an amide not an amine and is not considered basic.
Weakest based because it is an amide not an amine and is not considered basic.
Strongest base because it is the only aliphatic amine.
 Weakest based because it is an amide not an amine and is not considered basic.
Weakest based because it is an amide not an amine and is not considered basic. 4
Classify each of the nitrogen atoms in the compound shown below as primary, secondary, tertiary, or quaternary.
Classify:
Classify:


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
5
Give the major organic product(s) of each of the following reaction or sequence of reactions. Show all relevant stereochemistry.
Give major product(s):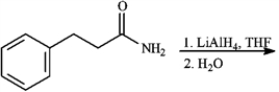
Give major product(s):


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
6
The pKa of the 4-methoxyanilinium ion is 5.34. What is the pKb for 4-methoxyaniline?

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
7
Give the major organic product(s) of each of the following reaction or sequence of reactions. Show all relevant stereochemistry.
Give major product(s):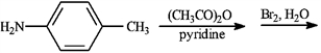
Give major product(s):
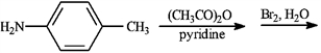

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
8
Rank the following compounds in order of increasing basicity. Label the least basic compound "1" and the most basic compound "4". Place the number corresponding to the compound's rank in the blank below the compound. 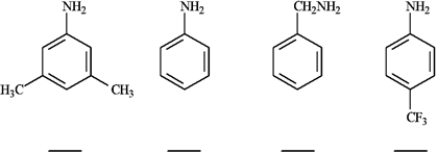


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
9
The pKa of aniline is 6.15. What percent exists in the neutral and protonated forms in a 0.00100 M solution of aniline at pH 5.75?

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
10
Give the major organic product(s) of each of the following reaction or sequence of reactions. Show all relevant stereochemistry.
Give major product(s):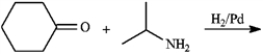
Give major product(s):


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
11
Consider the reaction below to answer the following question(s).
Methamphetamine can be synthesized by reacting phenyl-2-propanone with methylamine in the presence of H2/Ni.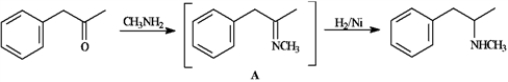
Refer to instructions. Intermediate A is an example of:
A) an imine
B) an enamine
C) an iminium ion
D) an imide
Methamphetamine can be synthesized by reacting phenyl-2-propanone with methylamine in the presence of H2/Ni.

Refer to instructions. Intermediate A is an example of:
A) an imine
B) an enamine
C) an iminium ion
D) an imide

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
12
Based on the following structures, name the compound.
Name: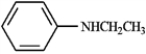
Name:


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
13
Give the major organic product(s) of each of the following reaction or sequence of reactions. Show all relevant stereochemistry.
Give major product(s):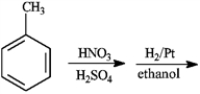
Give major product(s):


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
14
Based on the following structures, name the compound.
Name: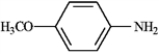
Name:


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
15
Draw structures corresponding to each of the IUPAC names given in the following question(s).
Draw:
N,N-dimethylcyclopentanamine
Draw:
N,N-dimethylcyclopentanamine

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
16
Draw structures corresponding to each of the IUPAC names given in the following question(s).
Draw:
hexane-1,6-diamine
Draw:
hexane-1,6-diamine

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
17
Based on the following structures, name the compound.
Name: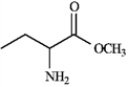
Name:


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
18
Refer to the table of pKas below to answer the following question.pKas of Some Arylammonium Ions 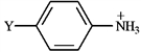

Refer to instructions. Based on the pKas for their corresponding ammonium ions, which arylamine above is the strongest base?


Refer to instructions. Based on the pKas for their corresponding ammonium ions, which arylamine above is the strongest base?

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
19
Consider the reaction below to answer the following question(s).
Methamphetamine can be synthesized by reacting phenyl-2-propanone with methylamine in the presence of H2/Ni.
Refer to instructions. Identify the nucleophile in the initial reaction of phenyl-2-propanone to yield intermediate A and classify the type of reaction that produces the final product from A.
Methamphetamine can be synthesized by reacting phenyl-2-propanone with methylamine in the presence of H2/Ni.

Refer to instructions. Identify the nucleophile in the initial reaction of phenyl-2-propanone to yield intermediate A and classify the type of reaction that produces the final product from A.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
20
Circle any of the following that would be classified as a heterocyclic amine. 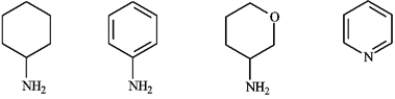


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
21
Predict the major organic product obtained from the following sequence of reactions. 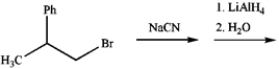


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
22
What is the major organic product obtained from the following reaction? 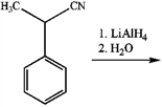
A)
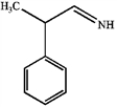
B)
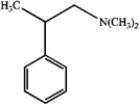
C)
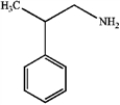
D)
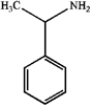

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
23
What is the IUPAC name of the following compound? 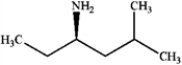
A) (R)-4-amino-2-methylhexane
B) (S)-4-amino-2-methylhexane
C) N-ethyl N-(3-methylpropyl) amine
D) (R)-3-amino-5-methylhexane

A) (R)-4-amino-2-methylhexane
B) (S)-4-amino-2-methylhexane
C) N-ethyl N-(3-methylpropyl) amine
D) (R)-3-amino-5-methylhexane

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
24
Consider the reaction below to answer the following questions. 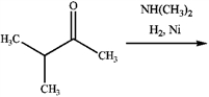 a)What is the major organic product obtained from the reaction?
a)What is the major organic product obtained from the reaction?
a.
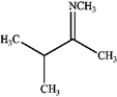
b.
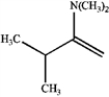
c.
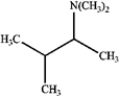
d.
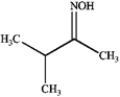
b)
How could IR spectroscopy be used to verify the identity of the product?
 a)What is the major organic product obtained from the reaction?
a)What is the major organic product obtained from the reaction? a.

b.

c.

d.

b)
How could IR spectroscopy be used to verify the identity of the product?

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
25
Give the major organic product(s) of each of the following reaction or sequence of reactions. Show all relevant stereochemistry.
Give major product(s):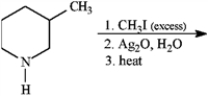
Give major product(s):


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following compounds are primary (1°) amines? 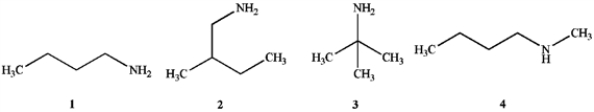
A) 1 and 2
B) 1 and 3
C) 1, 2 and 3
D) 1, 2, 3 and 4

A) 1 and 2
B) 1 and 3
C) 1, 2 and 3
D) 1, 2, 3 and 4

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
27
What is the major organic product obtained from the following reaction? 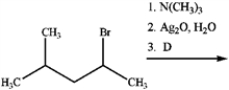
A)
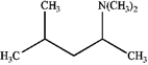
B)
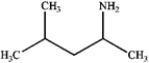
C)
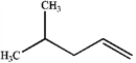
D)
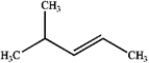

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
28
Give the major organic product(s) of each of the following reaction or sequence of reactions. Show all relevant stereochemistry.
Give major product(s):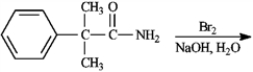
Give major product(s):


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
29
What is the correct assignment of the names of the following aromatic compounds? 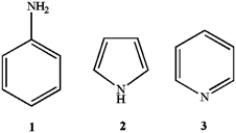
A) 1 = anisole; 2 = furan; 3 = pyrimidine
B) 1 = aniline; 2 = pyrrole; 3 = pyridine
C) 1 = anisole; 2 = pyridine; 3 = pyrrole
D) 1 = aniline; 2 = imidazole; 3 = pyridine

A) 1 = anisole; 2 = furan; 3 = pyrimidine
B) 1 = aniline; 2 = pyrrole; 3 = pyridine
C) 1 = anisole; 2 = pyridine; 3 = pyrrole
D) 1 = aniline; 2 = imidazole; 3 = pyridine

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
30
Spectral analysis of the following compound included an IR and 1H NMR. Which of the following would be evident in this analysis? 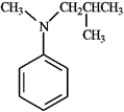
A) Lack of defining features in the 1H NMR
B) Strong singlet in the 1H NMR at about δ 2.2
C) Pair of bands in the IR between 3350 and 3450 cm−1
D) Single band in the IR between 3350 and 3450 cm−1
E) Both b and d

A) Lack of defining features in the 1H NMR
B) Strong singlet in the 1H NMR at about δ 2.2
C) Pair of bands in the IR between 3350 and 3450 cm−1
D) Single band in the IR between 3350 and 3450 cm−1
E) Both b and d

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
31
What is the m/z for the α-cleavage of the following amine? 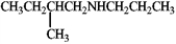

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
32
The IR spectrum below was obtained for an unknown compound known to be an amine. How would this compound be classified based on the IR spectrum? 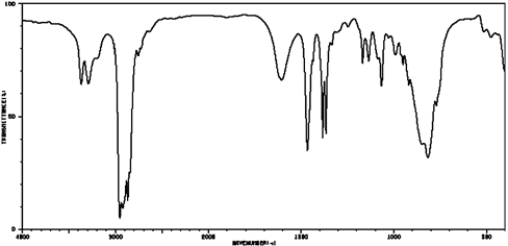
A) primary amine
B) secondary amine
C) tertiary amine
D) quaternary ammonium salt

A) primary amine
B) secondary amine
C) tertiary amine
D) quaternary ammonium salt

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
33
Circle any of the following compounds, that when used as the starting material and treated as shown below, would produce a single product. 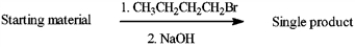
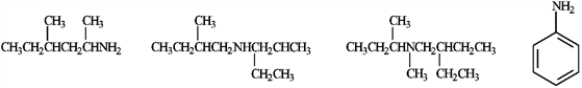
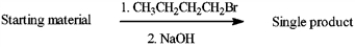
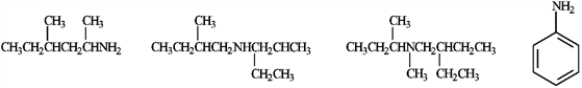

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following compounds are tertiary (3°) amines? 1.(CH3)2CHN(CH3)2
2)CH3CH(CH3)N(CH2CH3)2
3)CH3CH2CH2NHCH3
4)CH3CH2CH2CH2CH2NH2
A) 1 and 2
B) 1 and 3
C) 1, 2 and 3
D) 1, 2, 3 and 4
2)CH3CH(CH3)N(CH2CH3)2
3)CH3CH2CH2NHCH3
4)CH3CH2CH2CH2CH2NH2
A) 1 and 2
B) 1 and 3
C) 1, 2 and 3
D) 1, 2, 3 and 4

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
35
What is the major organic product obtained from the following reaction? 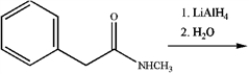
A)
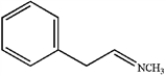
B)
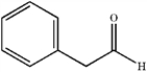
C)
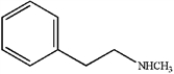
D)
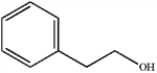

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
36
Propose a structure that is consistent with the following spectral data:
MS:
M+ at m/z = 77
IR:
3350 cm−1 (weak, single band)
1H NMR:
δ1.05 (br s, 1H), δ1.15 (t, 6H), δ2.65 (q, 4H)
MS:
M+ at m/z = 77
IR:
3350 cm−1 (weak, single band)
1H NMR:
δ1.05 (br s, 1H), δ1.15 (t, 6H), δ2.65 (q, 4H)

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck


