Deck 13: Alcohols, Phenols, and Thiols; Ethers and Sulfides
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/38
Play
Full screen (f)
Deck 13: Alcohols, Phenols, and Thiols; Ethers and Sulfides
1
To answer the following question(s), consider the reaction below. 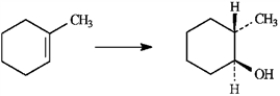
Refer to instructions. The alcohol product is classified as a:
A) 1° alcohol
B) 2° alcohol
C) 3° alcohol
D) 4° alcohol

Refer to instructions. The alcohol product is classified as a:
A) 1° alcohol
B) 2° alcohol
C) 3° alcohol
D) 4° alcohol
2° alcohol
2
Provide correct IUPAC names for each of the structures below.
Name: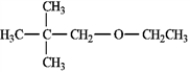
Name:
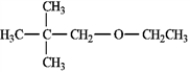
1-ethoxy-2,2-dimethylpropane or ethyl 2,2-dimethylpropyl ether
3
To answer the following question(s), consider the reaction below. 
Refer to instructions. Provide the IUPAC name for the product alcohol.

Refer to instructions. Provide the IUPAC name for the product alcohol.
trans-2-methylcyclohexanol
4
To answer the following question(s), consider the reaction below. 
Refer to instructions. Provide the complete IUPAC name for the starting material in this reaction and classify the alcohol as primary, secondary, or tertiary.

Refer to instructions. Provide the complete IUPAC name for the starting material in this reaction and classify the alcohol as primary, secondary, or tertiary.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
5
Draw structures corresponding to each of the following IUPAC names.
Draw:
3-methylbut-2-en-1-ol
Draw:
3-methylbut-2-en-1-ol

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
6
Consider the reaction below to answer the following question. 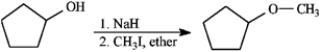
Refer to instructions. Mechanistically, the Williamson ether synthesis outlined above is:
A) an E1 process
B) an SN1 process
C) an E2 process
D) an SN2 process

Refer to instructions. Mechanistically, the Williamson ether synthesis outlined above is:
A) an E1 process
B) an SN1 process
C) an E2 process
D) an SN2 process

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
7
Consider the Grignard reaction below to answer the following question(s). 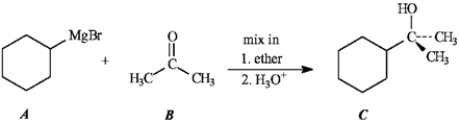
Predict the product of the following reaction.

Predict the product of the following reaction.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
8
Provide correct IUPAC names for each of the structures below.
Name: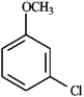
Name:


Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
9
Draw structures corresponding to each of the following IUPAC names.
Draw:
allyl benzyl ether
Draw:
allyl benzyl ether

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
10
Consider the Grignard reaction below to answer the following question(s). 
Refer to instructions. The nucleophile in this reaction is indicated by letter _____.
A)A
B)B
C)C

Refer to instructions. The nucleophile in this reaction is indicated by letter _____.
A)A
B)B
C)C

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
11
To answer the following question(s), consider the reaction below. 
Refer to instructions. The conversion of an alcohol into an alkyl chloride by reaction with SOCl2 is an example of:
A) an E1 process
B) an SN1 process
C) an E2 process
D) an SN2 process

Refer to instructions. The conversion of an alcohol into an alkyl chloride by reaction with SOCl2 is an example of:
A) an E1 process
B) an SN1 process
C) an E2 process
D) an SN2 process

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
12
Consider the Grignard reaction below to answer the following question(s). 
Refer to instructions. If a secondary alcohol were desired as a product of the reaction, B should be replaced with
A) an ester
B) an aldehyde
C) formaldehyde (methanal)
D) a primary alcohol

Refer to instructions. If a secondary alcohol were desired as a product of the reaction, B should be replaced with
A) an ester
B) an aldehyde
C) formaldehyde (methanal)
D) a primary alcohol

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
13
Draw structures corresponding to each of the following IUPAC names.
Draw:
2, 4, 6-trinitrophenol
Draw:
2, 4, 6-trinitrophenol

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
14
Draw structures corresponding to each of the following IUPAC names.
Draw:
3-methylbutane-1-thiol
Draw:
3-methylbutane-1-thiol

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
15
Draw structures corresponding to each of the following IUPAC names.
Draw:
2-phenylpropan-2-ol
Draw:
2-phenylpropan-2-ol

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
16
To answer the following question(s), consider the reaction below. 
Refer to instructions. On the templates provided below, draw both conformations of the alcohol product. Circle the least stable conformation.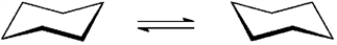

Refer to instructions. On the templates provided below, draw both conformations of the alcohol product. Circle the least stable conformation.


Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
17
Consider the Grignard reaction below to answer the following question(s). 
A useful and general method for the synthesis of alcohols is the addition of Grignard reagents to carbonyl compounds. Show what Grignard reagent and what carbonyl compound you would start with to prepare each alcohol below. List all possibilities.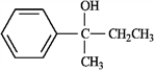

A useful and general method for the synthesis of alcohols is the addition of Grignard reagents to carbonyl compounds. Show what Grignard reagent and what carbonyl compound you would start with to prepare each alcohol below. List all possibilities.


Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following would not react with either Na2Cr2O7 or pyridinium chlorochromate? A 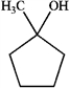 B
B 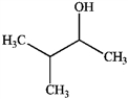 C
C 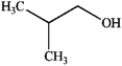 D
D 
A) only A
B) only C
C) only B and D
D) A, B, C, and D
 B
B  C
C  D
D 
A) only A
B) only C
C) only B and D
D) A, B, C, and D

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
19
Consider the Grignard reaction below to answer the following question(s). 
Refer to instructions. The electrophile in this reaction is indicated by letter _____.
A)A
B)B
C)C

Refer to instructions. The electrophile in this reaction is indicated by letter _____.
A)A
B)B
C)C

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
20
Draw structures corresponding to each of the following IUPAC names.
Draw:
cyclopropyl ethyl sulfide
Draw:
cyclopropyl ethyl sulfide

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
21
What is the IUPAC name of the following compound? 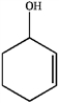
A) cyclohexen-3-ol
B) cyclohexen-2-ol
C) cyclohex-1-en-3-ol
D) cyclohex-2-en-1-ol

A) cyclohexen-3-ol
B) cyclohexen-2-ol
C) cyclohex-1-en-3-ol
D) cyclohex-2-en-1-ol

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
22
What is the IUPAC name of the following compound? 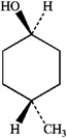
A) cis-4-methylcyclohexanol
B) trans-4-methylcyclohexanol
C) cis-4-hydroxy-1-methylcyclohexane
D) trans-4-hydroxy-1-methylcyclohexane

A) cis-4-methylcyclohexanol
B) trans-4-methylcyclohexanol
C) cis-4-hydroxy-1-methylcyclohexane
D) trans-4-hydroxy-1-methylcyclohexane

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is probably the least useful in IR characterization of a compound?
A) phenol: 3500 cm−1
B) alcohol: 3500 cm−1
C) phenol: 1500−1600 cm−1
D) ether: 1050−1150 cm−1
E) all of these are about equally useful
A) phenol: 3500 cm−1
B) alcohol: 3500 cm−1
C) phenol: 1500−1600 cm−1
D) ether: 1050−1150 cm−1
E) all of these are about equally useful

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following ethers cannot be prepared by a Williamson ether synthesis?
A) tert-butyl phenyl ether
B) isopropyl methyl ether
C) anisole
D) tert-butyl methyl ether
A) tert-butyl phenyl ether
B) isopropyl methyl ether
C) anisole
D) tert-butyl methyl ether

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
25
Predict the product of the following reaction. 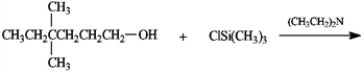
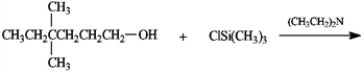

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
26
Predict the product in each step of the following reaction. 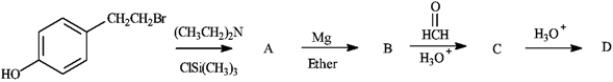


Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
27
What is the best choice of reagent to achieve the following transformation? 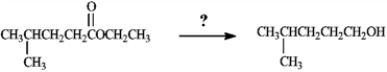
A) CrO3, H3O+, acetone
B) pyridium chlorochromate, CH2Cl2
C) SOCl2, pyridine
D) NaBH4, H3O+
E) LiAlH4, H3O+

A) CrO3, H3O+, acetone
B) pyridium chlorochromate, CH2Cl2
C) SOCl2, pyridine
D) NaBH4, H3O+
E) LiAlH4, H3O+

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
28
What is the IUPAC name of the following compound? 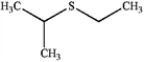
A) ethyl isopropyl thiol
B) 2-methylsulfanylpropane
C) ethyl isopropyl disulfide
D) ethyl isopropyl sulfide

A) ethyl isopropyl thiol
B) 2-methylsulfanylpropane
C) ethyl isopropyl disulfide
D) ethyl isopropyl sulfide

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
29
Addition of chlorotrimethylsilane to an alcohol
A) results in an SN1 reaction.
B) requires acidic reaction conditions.
C) is sterically hindered by the three methyl groups.
D) results in the formation of an ether.
E) produces a more reactive species.
A) results in an SN1 reaction.
B) requires acidic reaction conditions.
C) is sterically hindered by the three methyl groups.
D) results in the formation of an ether.
E) produces a more reactive species.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following are ethers? A 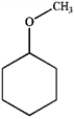 B
B 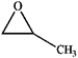 C
C 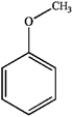 D
D 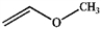
A) only A and B
B) only A and D
C) only A, B, and C
D) all of these are ethers
 B
B  C
C  D
D 
A) only A and B
B) only A and D
C) only A, B, and C
D) all of these are ethers

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
31
Identify the m/z peak for the alpha cleavage and the peak for the dehydration of the following alcohol. 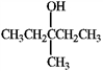
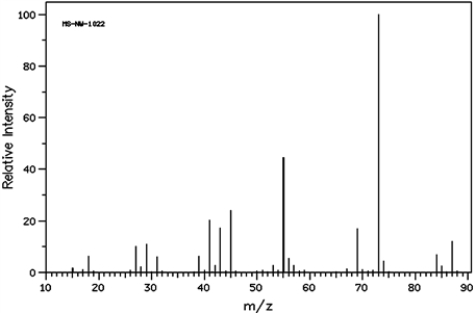



Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
32
The following IR spectrum would most likely be associated with 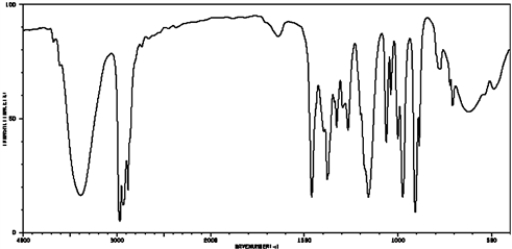
A) phenol
B) aromatic alcohol
C) aliphatic alcohol
D) ether
E) cannot determine from the isolated spectrum

A) phenol
B) aromatic alcohol
C) aliphatic alcohol
D) ether
E) cannot determine from the isolated spectrum

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
33
What is the major organic product obtained from the following reaction? 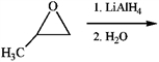
A)
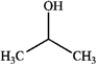
B)
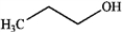
C)
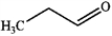
D)
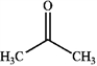
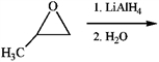
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
34
Predict the major products of the following by drawing the structures of A and B. 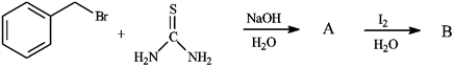
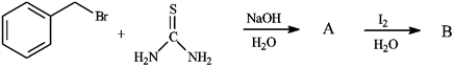

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is not a property of a protecting group?
A) change the reactivity of a functional group
B) inert to reaction conditions
C) becomes a permanent part of the product
D) alters the mechanism of the desired reaction
E) all of these are properties of a protecting group
A) change the reactivity of a functional group
B) inert to reaction conditions
C) becomes a permanent part of the product
D) alters the mechanism of the desired reaction
E) all of these are properties of a protecting group

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
36
How would you synthesize the following class of quinones (ubiquinones) from the corresponding hydroquinones? 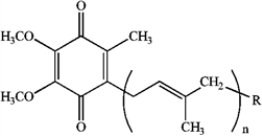


Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
37
Answer the following questions.
a)Identify the peak for the carbon indicted by the arrow on the 13C NMR spectra.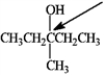
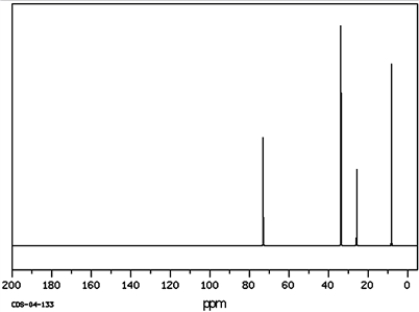
b)What type of signal would be expected in the 1H NMR spectrum for the hydrogen atom indicated with the arrow?
a. singlet
b. triplet
c. quintet
d. sextet
e. quartet of triplets (eight peaks)
a)Identify the peak for the carbon indicted by the arrow on the 13C NMR spectra.


b)What type of signal would be expected in the 1H NMR spectrum for the hydrogen atom indicated with the arrow?
a. singlet
b. triplet
c. quintet
d. sextet
e. quartet of triplets (eight peaks)

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
38
What is the best way to make the following ether by a Williamson ether synthesis? 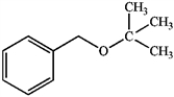


Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck


