Deck 11: Structure Determination: Nuclear Magnetic Resonance Spectroscopy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/41
Play
Full screen (f)
Deck 11: Structure Determination: Nuclear Magnetic Resonance Spectroscopy
1
To answer the following questions, consider the data and 1H NMR spectrum below.
The mass spectrum of this compound shows a molecular ion at m/z = 113, the IR spectrum has characteristic absorptions at 2270 and 1735 cm−1, and the 13C NMR spectrum has five signals.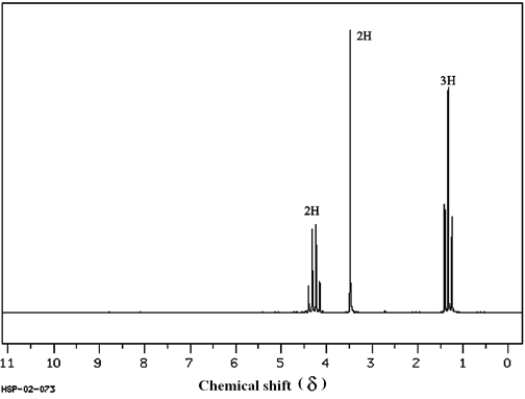 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
Refer to instructions. How many types of nonequivalent protons are there in this molecule?
The mass spectrum of this compound shows a molecular ion at m/z = 113, the IR spectrum has characteristic absorptions at 2270 and 1735 cm−1, and the 13C NMR spectrum has five signals.
 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)Refer to instructions. How many types of nonequivalent protons are there in this molecule?
three
2
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband decoupled 13C NMR spectra.
Number of signals: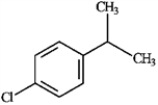
Number of signals:

six 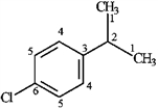

3
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband decoupled 13C NMR spectra.
Number of signals:
Number of signals:

five 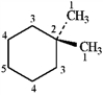

4
Predict the splitting patterns you would expect for each proton in the molecules below:
Predict: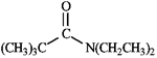
Predict:


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following would not produce nuclear magnetic resonance?
A) "2H"
B) "14N"
C) "16O"
D) "19F"
A) "2H"
B) "14N"
C) "16O"
D) "19F"

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
6
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband decoupled 13C NMR spectra.
Number of signals: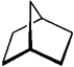
Number of signals:


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
7
Refer to the structure of 3-methylbutan-2-one below to answer the following question(s). 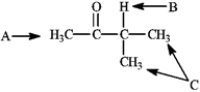
A compound with the molecular formula C5H12O produces only two singlets in the 1H NMR spectrum.
a)Propose a structure for this compound.
b)How many signals would be present in the 13C NMR for this structure?

A compound with the molecular formula C5H12O produces only two singlets in the 1H NMR spectrum.
a)Propose a structure for this compound.
b)How many signals would be present in the 13C NMR for this structure?

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
8
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband decoupled 13C NMR spectra.
Which of the following compounds would produce the most downfield signal in a 13C NMR spectrum? A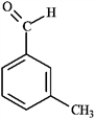 B
B 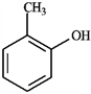 C
C 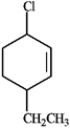 D
D 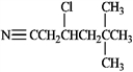
A) A
B) B
C) C
D) D
Which of the following compounds would produce the most downfield signal in a 13C NMR spectrum? A
 B
B  C
C  D
D 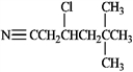
A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
9
Nuclear magnetic resonance spectroscopy provides information about a molecule's:
A) conjugated pi electron system.
B) size and formula.
C) carbon-hydrogen framework.
D) functional groups.
A) conjugated pi electron system.
B) size and formula.
C) carbon-hydrogen framework.
D) functional groups.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
10
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband decoupled 13C NMR spectra.
Which of the following would produce only singlets in an 1H NMR spectrum? A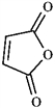 B
B 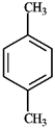 C
C 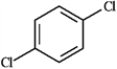 D
D 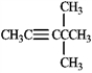
A) A
B) B
C) C
D) D
E) all of these produce only singlets
Which of the following would produce only singlets in an 1H NMR spectrum? A
 B
B  C
C  D
D 
A) A
B) B
C) C
D) D
E) all of these produce only singlets

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
11
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband decoupled 13C NMR spectra.
Arrange the following compounds in order of increasing number of signals in a 13C NMR spectrum.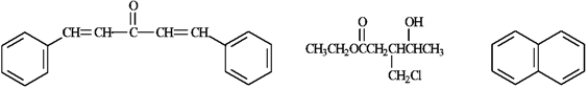
Arrange the following compounds in order of increasing number of signals in a 13C NMR spectrum.


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
12
Refer to the structure of 3-methylbutan-2-one below to answer the following question(s). 
Refer to instructions. What is the splitting pattern for the hydrogens in 3-methylbutan-2-one labeled A, B, and C, respectively?
A) singlet, singlet, singlet
B) singlet, septet, quartet
C) singlet, septet, doublet
D) singlet, septet, doublet, doublet

Refer to instructions. What is the splitting pattern for the hydrogens in 3-methylbutan-2-one labeled A, B, and C, respectively?
A) singlet, singlet, singlet
B) singlet, septet, quartet
C) singlet, septet, doublet
D) singlet, septet, doublet, doublet

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
13
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband decoupled 13C NMR spectra.
Number of signals: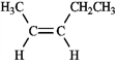
Number of signals:


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
14
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband decoupled 13C NMR spectra.
Number of signals: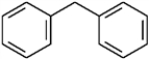
Number of signals:


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
15
Refer to the structure of 3-methylbutan-2-one below to answer the following question(s). 
Treatment of tert-butyl alcohol with hydrogen chloride yields a mixture of tert-butyl chloride and 2-methylpropene.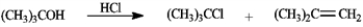 a)After chromatographic separation, how would you use 1H NMR to help you decide which was which?
a)After chromatographic separation, how would you use 1H NMR to help you decide which was which?
b)How would the 13C NMR for the two compounds differ?

Treatment of tert-butyl alcohol with hydrogen chloride yields a mixture of tert-butyl chloride and 2-methylpropene.
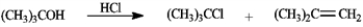 a)After chromatographic separation, how would you use 1H NMR to help you decide which was which?
a)After chromatographic separation, how would you use 1H NMR to help you decide which was which?b)How would the 13C NMR for the two compounds differ?

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
16
Refer to the structure of 3-methylbutan-2-one below to answer the following question(s). 
A compound with the molecular formula C6H4ClBr produces only two doublets in the 1H NMR spectrum.
a)Propose a structure for this compound.
b)How many signals would be present in the 13CNMR for this structure?

A compound with the molecular formula C6H4ClBr produces only two doublets in the 1H NMR spectrum.
a)Propose a structure for this compound.
b)How many signals would be present in the 13CNMR for this structure?

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
17
Explain why all protons in a molecule do not absorb rf energy at the same frequency.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
18
Predict the splitting patterns you would expect for each proton in the molecules below:
Predict: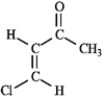
Predict:


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
19
To answer the following questions, consider the data and 1H NMR spectrum below.
The mass spectrum of this compound shows a molecular ion at m/z = 113, the IR spectrum has characteristic absorptions at 2270 and 1735 cm−1, and the 13C NMR spectrum has five signals. (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
Refer to instructions. Based on the mass spectral data and the IR data, what functional groups are present in this compound?
The mass spectrum of this compound shows a molecular ion at m/z = 113, the IR spectrum has characteristic absorptions at 2270 and 1735 cm−1, and the 13C NMR spectrum has five signals.
 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)Refer to instructions. Based on the mass spectral data and the IR data, what functional groups are present in this compound?

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
20
Refer to the structure of 3-methylbutan-2-one below to answer the following question(s). 
Refer to instructions. What is the ratio of peak areas upon integration of the spectrum for A, B, and C respectively?
A) 3:1:3:3
B) 1:1:6
C) 1:1:6.0
D) 3:1:6

Refer to instructions. What is the ratio of peak areas upon integration of the spectrum for A, B, and C respectively?
A) 3:1:3:3
B) 1:1:6
C) 1:1:6.0
D) 3:1:6

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
21
How many signals appear in the broadband-decoupled 13C NMR spectrum of 1,3-dibromobenzene?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
22
Answer the following question(s) for the compound whose 1H NMR spectra is shown below.
C4H8O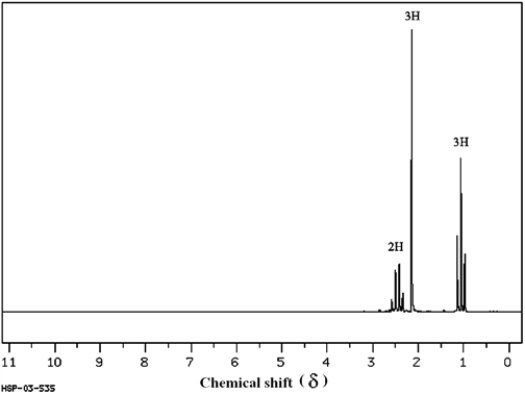 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
Refer to instructions. Propose a structure for this compound.
C4H8O
 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)Refer to instructions. Propose a structure for this compound.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
23
Answer the following question(s) for the compound whose 1H NMR spectra is shown below.
C4H8O (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
Refer to instructions. Describe each signal in terms of its integration, splitting and chemical shift.
C4H8O
 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)Refer to instructions. Describe each signal in terms of its integration, splitting and chemical shift.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
24
Which feature in the 1H NMR spectrum provides information about the electronic environment of the protons in a compound?
A) number of signals
B) integration of signals
C) splitting of signals
D) chemical shift
A) number of signals
B) integration of signals
C) splitting of signals
D) chemical shift

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
25
The following questions pertain to the display of NMR spectra. Match a term to each description below.
The exact place on the spectrum at which a nucleus absorbs is called its _____.
A)TMS
B)high-field or upfield side
C)MHz
D)delta (δ)
E)low-field or downfield side
F)chemical shift
G)intensity
The exact place on the spectrum at which a nucleus absorbs is called its _____.
A)TMS
B)high-field or upfield side
C)MHz
D)delta (δ)
E)low-field or downfield side
F)chemical shift
G)intensity

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
26
If the proton attached to C2 in 1,1,2-tribromopropane is coupled with the protons on C1 (J = 3.5 Hz) and C3 (J = 6.8), draw the tree diagram of the C2 proton and predict the splitting pattern. 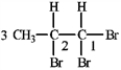


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
27
How many positive and negative peaks appear in the DEPT-135 and in the DEPT-90 spectrum of 2-methylpentane?
A) DEPT-135: two positive and one negative, DEPT-90: one positive
B) DEPT-135: three positive and two negative, DEPT-90: one positive
C) DEPT-135: three positive and two negative, DEPT-90: no signals
D) DEPT-135: two positive and three negative, DEPT-90: two positive
A) DEPT-135: two positive and one negative, DEPT-90: one positive
B) DEPT-135: three positive and two negative, DEPT-90: one positive
C) DEPT-135: three positive and two negative, DEPT-90: no signals
D) DEPT-135: two positive and three negative, DEPT-90: two positive

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following compounds gives a 1H NMR spectrum consisting of only two singlets?
A) CH3OCH2CH2OCH2CH3
B) CH3OCH2CH2CH2CH2OH
C) CH3OC(CH3)2OCH3
D) CH3OCH2CH(CH3)OCH3
A) CH3OCH2CH2OCH2CH3
B) CH3OCH2CH2CH2CH2OH
C) CH3OC(CH3)2OCH3
D) CH3OCH2CH(CH3)OCH3

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following combinations of peaks appears in the 1H NMR spectrum of diethyl ether, CH3CH2OCH2CH3?
A) a triplet and a doublet
B) a quartet and a sextet
C) two singlets
D) a triplet and a quartet
A) a triplet and a doublet
B) a quartet and a sextet
C) two singlets
D) a triplet and a quartet

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following combinations of peaks appears in the 1H NMR spectrum of 2-methylpropane?
A) two singlets
B) a singlet and a nonet
C) a singlet and a decet
D) a doublet and a decet
A) two singlets
B) a singlet and a nonet
C) a singlet and a decet
D) a doublet and a decet

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
31
How many sets of equivalent protons are there in hexane, 2-methylhexane, and 3-methylhexane, respectively?

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
32
How many signals appear in the broadband-decoupled 13C NMR spectrum of 1,2-dibromobenzene?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
33
The following questions pertain to the display of NMR spectra. Match a term to each description below.
When looking at an NMR spectrum the right-hand part of the chart is the _____.
A)TMS
B)high-field or upfield side
C)MHz
D)delta (δ)
E)low-field or downfield side
F)chemical shift
G)intensity
When looking at an NMR spectrum the right-hand part of the chart is the _____.
A)TMS
B)high-field or upfield side
C)MHz
D)delta (δ)
E)low-field or downfield side
F)chemical shift
G)intensity

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
34
The following questions pertain to the display of NMR spectra. Match a term to each description below.
The NMR spectrum are calibrated using an arbitrary scale that is divided into _____ units.
A)TMS
B)high-field or upfield side
C)MHz
D)delta (δ)
E)low-field or downfield side
F)chemical shift
G)intensity
The NMR spectrum are calibrated using an arbitrary scale that is divided into _____ units.
A)TMS
B)high-field or upfield side
C)MHz
D)delta (δ)
E)low-field or downfield side
F)chemical shift
G)intensity

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
35
How many positive and negative peaks appear in the DEPT-135 spectrum of 2,4-dimethylpentane?
A) two positive and one negative
B) three positive and two negative
C) four positive and three negative
D) six positive and one negative
A) two positive and one negative
B) three positive and two negative
C) four positive and three negative
D) six positive and one negative

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following compounds gives a 1H NMR spectrum consisting of only a singlet and two signals in the 13C NMR?
A) 1,1-dibromopropane
B) 1,2-dibromopropane
C) 1,3-dibromopropane
D) 2,2-dibromopropane
A) 1,1-dibromopropane
B) 1,2-dibromopropane
C) 1,3-dibromopropane
D) 2,2-dibromopropane

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
37
Which feature in the 1H NMR spectrum provides information about the number of neighboring protons of each proton in the compound?
A) number of signals
B) integration of signals
C) splitting of signals
D) chemical shift
A) number of signals
B) integration of signals
C) splitting of signals
D) chemical shift

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
38
The following questions pertain to the display of NMR spectra. Match a term to each description below.
The calibration standard for 1H and 13C NMR is _____.
A)TMS
B)high-field or upfield side
C)MHz
D)delta (δ)
E)low-field or downfield side
F)chemical shift
G)intensity
The calibration standard for 1H and 13C NMR is _____.
A)TMS
B)high-field or upfield side
C)MHz
D)delta (δ)
E)low-field or downfield side
F)chemical shift
G)intensity

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
39
Which structure of molecular formula C4H8Cl2 fits the 1H NMR and 13C NMR spectra shown below? 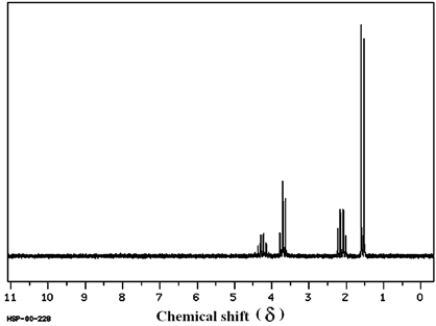
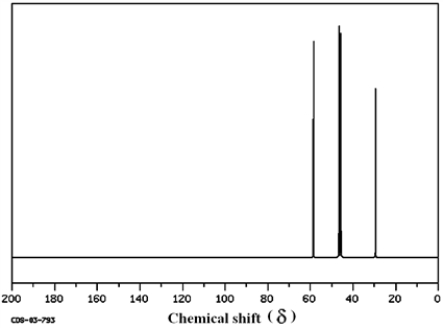 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
A)
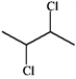
B)
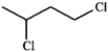
C)
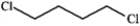
D)
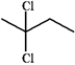

 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)A)

B)

C)

D)


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
40
Which feature in the 1H NMR spectrum provides information about the relative numbers of hydrogen atoms of each type found in a compound?
A) number of signals
B) integration of signals
C) splitting of signals
D) chemical shift
A) number of signals
B) integration of signals
C) splitting of signals
D) chemical shift

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
41
The following questions pertain to the display of NMR spectra. Match a term to each description below.
The vertical axis of spectrum displays the _____ of the signal.
A)TMS
B)high-field or upfield side
C)MHz
D)delta (δ)
E)low-field or downfield side
F)chemical shift
G)intensity
The vertical axis of spectrum displays the _____ of the signal.
A)TMS
B)high-field or upfield side
C)MHz
D)delta (δ)
E)low-field or downfield side
F)chemical shift
G)intensity

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck


