Deck 9: Aromatic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/37
Play
Full screen (f)
Deck 9: Aromatic Compounds
1
Which of the following sets of substituents are all deactivating groups in electrophilic aromatic substitution reactions?
A) Cl, CN, NO2
B) Cl, NH2, CH3
C) CH3, OCH3, COCH3
D) CH3, NH2, OCH3
A) Cl, CN, NO2
B) Cl, NH2, CH3
C) CH3, OCH3, COCH3
D) CH3, NH2, OCH3
Cl, CN, NO2
2
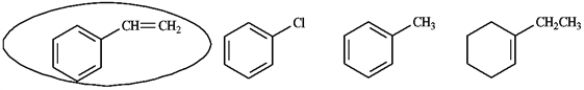
Circle the compound(s) in the following set that will undergo both substitution and addition when treated with Br2. 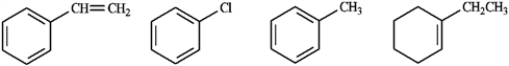

3
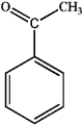
What is the major organic product obtained from the following reaction? 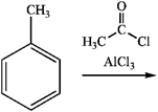
A)
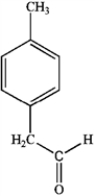
B)
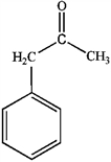
C)
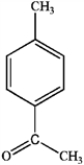
D)

A)

B)

C)

D)


4
Draw the structure of 4-bromo-2-fluorotoluene.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
5
What is the IUPAC name of the following compound? 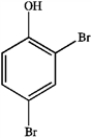
A) 2,4-dibromotoluene
B) 2,4-dibromophenol
C) 2,4-dibromoaniline
D) 4,6-dibromophenol

A) 2,4-dibromotoluene
B) 2,4-dibromophenol
C) 2,4-dibromoaniline
D) 4,6-dibromophenol

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following undergoes the most rapid acylation upon treatment with acetyl chloride and AlCl3?
A) benzene
B) toluene
C) chlorobenzene
D) 1,4-dichlorobenzene
A) benzene
B) toluene
C) chlorobenzene
D) 1,4-dichlorobenzene

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following would be an o/p-director in a nucleophilic aromatic substitution reaction?
A) −CH3
B) −OH
C) −F
D) −CN
E) all of these
A) −CH3
B) −OH
C) −F
D) −CN
E) all of these

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
8
Predict the major product of each of the following reactions. 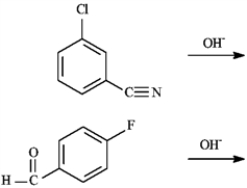


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
9
How many π electrons are present in the following heterocycle? 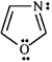
A) 2
B) 4
C) 6
D) 8
E) 10

A) 2
B) 4
C) 6
D) 8
E) 10

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following undergoes the most rapid bromination upon treatment with Br2/FeBr3?
A) benzene
B) nitrobenzene
C) bromobenzene
D) benzaldehyde
A) benzene
B) nitrobenzene
C) bromobenzene
D) benzaldehyde

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following compounds is aromatic?
A) ethane
B) cyclobuta-1,3-diene
C) benzene
D) cycloocta-1,3,5,7-tetraene
A) ethane
B) cyclobuta-1,3-diene
C) benzene
D) cycloocta-1,3,5,7-tetraene

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following does not correctly characterize a nucleophilic aromatic substitution reaction?
A) favored by electron-withdrawing groups
B) carbanion intermediates
C) −NO2 and −CHO activate the ring
D) −NO2 and −CHO are o/p-directors
E) an H atom is replaced on the ring
A) favored by electron-withdrawing groups
B) carbanion intermediates
C) −NO2 and −CHO activate the ring
D) −NO2 and −CHO are o/p-directors
E) an H atom is replaced on the ring

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements is true about the structure of benzene?
A) six atomic 2p orbitals interact to form six π molecular orbitals
B) there are three bonding π molecular orbitals and three π antibonding molecular orbitals
C) the ground state electronic configuration of benzene has six electrons in three π bonding molecular orbitals
D) the carbon−carbon bonds are all the same length
E) All of these
A) six atomic 2p orbitals interact to form six π molecular orbitals
B) there are three bonding π molecular orbitals and three π antibonding molecular orbitals
C) the ground state electronic configuration of benzene has six electrons in three π bonding molecular orbitals
D) the carbon−carbon bonds are all the same length
E) All of these

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
14
Consider the production of 1-bromo-4-nitrobenzene: 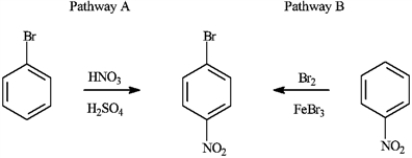 a)Explain why Pathway A for the production of 1-bromo-4-nitrobenzene produces a higher yield of the product.
a)Explain why Pathway A for the production of 1-bromo-4-nitrobenzene produces a higher yield of the product.
b)If reaction Pathway A is carried out at high temperatures, a trisubstituted product forms. Draw the structure of the product and explain why it forms.
 a)Explain why Pathway A for the production of 1-bromo-4-nitrobenzene produces a higher yield of the product.
a)Explain why Pathway A for the production of 1-bromo-4-nitrobenzene produces a higher yield of the product.b)If reaction Pathway A is carried out at high temperatures, a trisubstituted product forms. Draw the structure of the product and explain why it forms.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following sets of substituents are all o/p-directing in electrophilic aromatic substitution reactions?
A) Cl, CH3, CN
B) Br, OH, COCH3
C) Cl, OH, CH3
D) CN, NO2, COCH3
A) Cl, CH3, CN
B) Br, OH, COCH3
C) Cl, OH, CH3
D) CN, NO2, COCH3

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
16
Draw the resonance structures for acenaphthene. 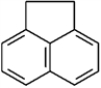


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following represents the energy levels of the molecular orbitals of benzene?
A)
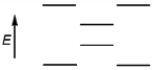
B)
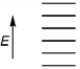
C)
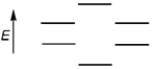
D)

A)
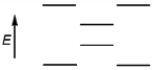
B)

C)
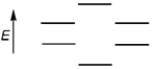
D)


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following statements correctly characterizes the following compound? 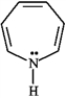
A) contains 6 π electrons and is aromatic
B) contains 6 π electrons and is nonaromatic
C) contains 8 π electrons and is antiaromatic
D) contains 8 π electrons and is aromatic
E) contains 8 π electrons and is nonaromatic

A) contains 6 π electrons and is aromatic
B) contains 6 π electrons and is nonaromatic
C) contains 8 π electrons and is antiaromatic
D) contains 8 π electrons and is aromatic
E) contains 8 π electrons and is nonaromatic

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following heterocycles is aromatic?
A)
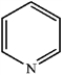
B)
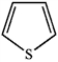
C)
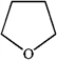
D)
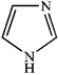
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
20
What is the IUPAC name of the following compound? 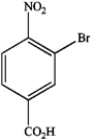
A) 3-bromo-4-nitrobenzaldehyde
B) 2-bromo-1-nitro-4-benzoic acid
C) 3-bromo-4-nitroacetophenone
D) 3-bromo-4-nitrobenzoic acid

A) 3-bromo-4-nitrobenzaldehyde
B) 2-bromo-1-nitro-4-benzoic acid
C) 3-bromo-4-nitroacetophenone
D) 3-bromo-4-nitrobenzoic acid

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
21
Place the following in order of reactivity towards electrophilic aromatic substitution. 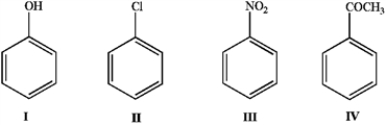
A) I > II > III > IV
B) I > II > IV > III
C) II > I > III > IV
D) II > I > IV > III
E) III > IV > II > I

A) I > II > III > IV
B) I > II > IV > III
C) II > I > III > IV
D) II > I > IV > III
E) III > IV > II > I

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
22
What is the major organic product obtained from the following reaction? 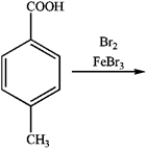
A)
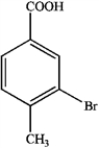
B)
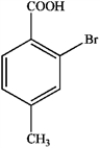
C)
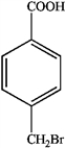
D)

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
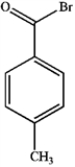
23
What is the major organic product obtained from the following reaction? 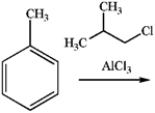
A)
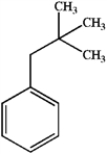
B)
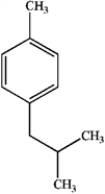
C)
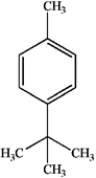
D)
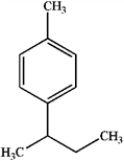

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
24
What is the major organic product obtained from the following sequence of reactions? 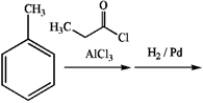
A)
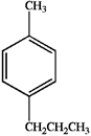
B)
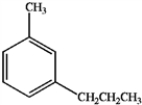
C)
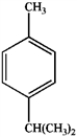
D)
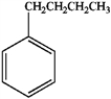

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
25
An accurate description of the structure of benzene is:
A) The π bonds are quickly moving around the ring.
B) There are two distinct structures that are in equilibrium.
C) All the carbon−carbon bonds are equal in length.
D) There are distinct single and double bonds.
E) Some bonds are longer than others.
A) The π bonds are quickly moving around the ring.
B) There are two distinct structures that are in equilibrium.
C) All the carbon−carbon bonds are equal in length.
D) There are distinct single and double bonds.
E) Some bonds are longer than others.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
26
Predict the major product of the following reaction: 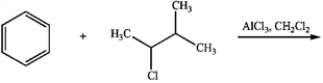


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
27
What is the electrophile in the reaction of benzene with acetyl chloride, CH3COCl, and AlCl3?
A) CH3C
O+
B) CH3+
C) CH3CO2−
D) benzene
A) CH3C
O+
B) CH3+
C) CH3CO2−
D) benzene

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
28
Consider the reaction below to answer the following question(s). 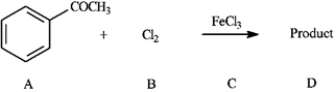
Refer to instructions. This reaction proceeds _____________ (faster or slower) than benzene.

Refer to instructions. This reaction proceeds _____________ (faster or slower) than benzene.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
29
Draw the major product of the following reaction: 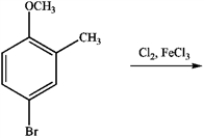


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the reaction below to answer the following question(s). 
Refer to instructions. The nucleophile in the reaction is indicated by letter _____.
A)A
B)B
C)C
D)D

Refer to instructions. The nucleophile in the reaction is indicated by letter _____.
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
31
What is the major organic product obtained from the following reaction? 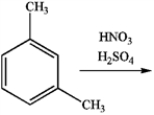
A)
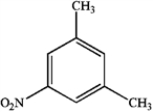
B)
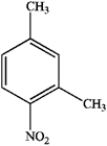
C)
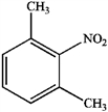
D)
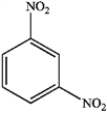

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
32
Consider the following compound: 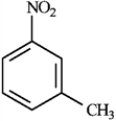 a)Provide the correct IUPAC name for the compound.
a)Provide the correct IUPAC name for the compound.
b)Draw and name the other isomeric nitrotoluenes.
 a)Provide the correct IUPAC name for the compound.
a)Provide the correct IUPAC name for the compound.b)Draw and name the other isomeric nitrotoluenes.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
33
Consider the following reaction: 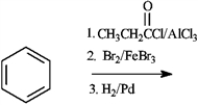 a)Predict the major product of the reaction.
a)Predict the major product of the reaction.
b)If steps 2 and 3 were reversed, what effect would this have on the major product?
 a)Predict the major product of the reaction.
a)Predict the major product of the reaction.b)If steps 2 and 3 were reversed, what effect would this have on the major product?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is a correct statement regarding electrophilic aromatic substitution?
A) The carbocation intermediate will lose a proton to regain aromaticity, usually from a position other than the site of electrophilic attack.
B) Formation of the carbocation intermediate has a high activation barrier due to loss of aromaticity.
C) The carbocation intermediate has several resonance structures and is negatively charged.
D) Re-formation of the aromatic ring has a low activation barrier and therefore occurs slowly.
E) Many suitable electrophiles are unreactive and can be stored for long periods of time prior to use.
A) The carbocation intermediate will lose a proton to regain aromaticity, usually from a position other than the site of electrophilic attack.
B) Formation of the carbocation intermediate has a high activation barrier due to loss of aromaticity.
C) The carbocation intermediate has several resonance structures and is negatively charged.
D) Re-formation of the aromatic ring has a low activation barrier and therefore occurs slowly.
E) Many suitable electrophiles are unreactive and can be stored for long periods of time prior to use.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
35
Consider the following sequence of reactions: 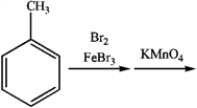
a) What is the major organic product obtained from the sequence of reactions?
a.
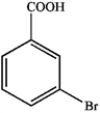
b.
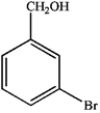
c.
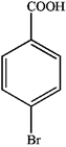
d.
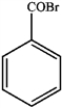
b) If KMnO4 had been replaced by Na2Cr2O7, what would be the final product of the reaction?

a) What is the major organic product obtained from the sequence of reactions?
a.

b.

c.

d.

b) If KMnO4 had been replaced by Na2Cr2O7, what would be the final product of the reaction?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
36
Consider the reaction below to answer the following question(s). 
Refer to instructions. The Lewis acid catalyst in the reaction is indicated by letter _____.
A)A
B)B
C)C
D)D

Refer to instructions. The Lewis acid catalyst in the reaction is indicated by letter _____.
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the reaction below to answer the following question(s). 
Refer to instructions. Draw the structure of product D.

Refer to instructions. Draw the structure of product D.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck


