Deck 6: An Overview of Organic Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/39
Play
Full screen (f)
Deck 6: An Overview of Organic Reactions
1
Identify the functional groups present in each compound below, and predict the direction of polarity in each.
Identify and predict:
amphetamine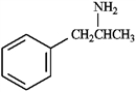
Identify and predict:
amphetamine

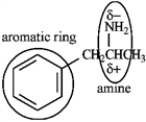
2
Classify each structure below as a nucleophile or electrophile, and briefly explain your choice.
Classify and explain:
azide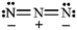
Classify and explain:
azide
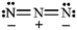
Azide ion is a nucleophile because it has a net negative charge (and lots of nonbonding electron electron pairs!).
3
Polarizability:
A) explains a nonpolar carbon−sulfur bond participating in polar reaction.
B) indicates the magnitude of a dipole moment.
C) is larger for smaller atoms with few electrons.
D) is the electric field associated with a polar bond.
E) all of these
A) explains a nonpolar carbon−sulfur bond participating in polar reaction.
B) indicates the magnitude of a dipole moment.
C) is larger for smaller atoms with few electrons.
D) is the electric field associated with a polar bond.
E) all of these
explains a nonpolar carbon−sulfur bond participating in polar reaction.
4
Use the first step of the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following question(s). 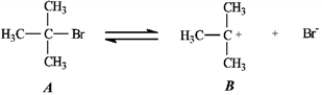
In the following generic reaction between a halogen (X) and an alkane (R), which of the following steps occurs slowly due to low concentration of reactants?
A)X⎯X → 2X∙
B)X∙ + R → HX + R∙
C)R∙ + X⎯X → RX + X∙
D)X∙ + R∙ → RX
E)X∙ + X∙ → X⎯X
F)R∙ + R∙ → R⎯R

In the following generic reaction between a halogen (X) and an alkane (R), which of the following steps occurs slowly due to low concentration of reactants?
A)X⎯X → 2X∙
B)X∙ + R → HX + R∙
C)R∙ + X⎯X → RX + X∙
D)X∙ + R∙ → RX
E)X∙ + X∙ → X⎯X
F)R∙ + R∙ → R⎯R

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following correctly compares the two elements in terms of polarizability?
A) S > O
B) F > Br
C) N > P
D) Cl > I
A) S > O
B) F > Br
C) N > P
D) Cl > I

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
6
Add curved arrows to the following reaction(s) to indicate the flow of electrons in each.
Indicate flow: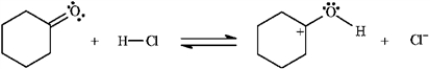
Indicate flow:
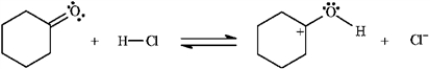

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
7
Use the first step of the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following question(s). 
Refer to instructions. Add curved arrows to indicate electron flow in the first step.

Refer to instructions. Add curved arrows to indicate electron flow in the first step.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
8
The reaction below is commonly used as a laboratory preparation of cyclohexene. Use this reaction to answer the following question(s). 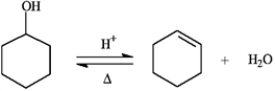
Refer to instructions. If this reaction under a given set of conditions has a Keq value of 5.67, what percent conversion to the product would be expected?
A) 85%
B) 5.67%
C) 17%
D) 15%
E) nearly 100%

Refer to instructions. If this reaction under a given set of conditions has a Keq value of 5.67, what percent conversion to the product would be expected?
A) 85%
B) 5.67%
C) 17%
D) 15%
E) nearly 100%

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
9
In theory, upon reaction with water in the presence of a strong acid, which of the following will produce more than one isomeric product?
A)

B)
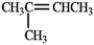
C)
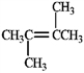
D)
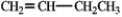
E) Both b and d
A)

B)
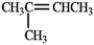
C)

D)
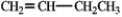
E) Both b and d

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
10
Identify and label the nucleophile and electrophile in each reaction below.
Identify and label: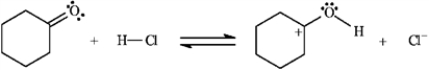
Identify and label:
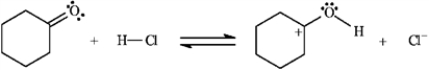

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
11
Use the first step of the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following question(s). 
In the following generic reaction between a halogen (X) and an alkane (R), which of the following steps would be considered a chain termination step?
A)X⎯X → 2X∙
B)X∙ + R → HX + R∙
C)R∙ + X⎯X → RX + X∙
D)X∙ + R∙ → RX
E)X∙ + X∙ → X⎯X
F)R∙ + R∙ → R⎯R

In the following generic reaction between a halogen (X) and an alkane (R), which of the following steps would be considered a chain termination step?
A)X⎯X → 2X∙
B)X∙ + R → HX + R∙
C)R∙ + X⎯X → RX + X∙
D)X∙ + R∙ → RX
E)X∙ + X∙ → X⎯X
F)R∙ + R∙ → R⎯R

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
12
How many monochlorosubstitution products are possible for 2,3-dimethylbutane?
A) 1
B) 2
C) 4
D) 5
E) 6
A) 1
B) 2
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
13
Classify each structure below as a nucleophile or electrophile, and briefly explain your choice.
Classify and explain:
phenol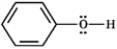
Classify and explain:
phenol


Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
14
In an organic reaction, which of the following is most likely to function as only a nucleophile?
A) BF3
B) (CH3)2CH2NH2
C) Fe2+
D) CH3CH2S−
E) both a and c
A) BF3
B) (CH3)2CH2NH2
C) Fe2+
D) CH3CH2S−
E) both a and c

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
15
The reaction below is commonly used as a laboratory preparation of cyclohexene. Use this reaction to answer the following question(s). 
Refer to instructions. The forward and reverse reactions are classified, respectively, as:
A) addition, elimination
B) elimination, substitution
C) elimination, addition
D) elimination, rearrangement
E) substitution, addition

Refer to instructions. The forward and reverse reactions are classified, respectively, as:
A) addition, elimination
B) elimination, substitution
C) elimination, addition
D) elimination, rearrangement
E) substitution, addition

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
16
Use the first step of the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following question(s). 
Refer to instructions. Species B is:
A) a carbocation
B) a radical
C) a carbanion
D) a free radical

Refer to instructions. Species B is:
A) a carbocation
B) a radical
C) a carbanion
D) a free radical

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
17
Consider the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following question(s). 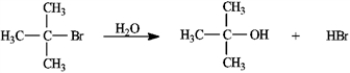
Refer to instructions. This reaction is an example of:
A) a substitution reaction.
B) a rearrangement reaction.
C) an elimination reaction.
D) an addition reaction.

Refer to instructions. This reaction is an example of:
A) a substitution reaction.
B) a rearrangement reaction.
C) an elimination reaction.
D) an addition reaction.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is a characteristic of a polar reaction?
A) symmetrical bond making and breaking
B) one electron from each reactant forms the bond
C) involves a neutral species with an unpaired electron
D) are more common that radical reactions
E) all of these
A) symmetrical bond making and breaking
B) one electron from each reactant forms the bond
C) involves a neutral species with an unpaired electron
D) are more common that radical reactions
E) all of these

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
19
Identify the functional groups present in each compound below, and predict the direction of polarity in each.
Identify and predict:
mustard gas Cl−CH2CH2−S−CH2CH2−Cl
Identify and predict:
mustard gas Cl−CH2CH2−S−CH2CH2−Cl

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
20
Identify and label the nucleophile and electrophile in each reaction below.
Identify and label:
Identify and label:


Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
21
Add curved arrows to the following reaction(s) to indicate the flow of electrons in each.
The structures below show the stepwise bond making and bond breaking in this reaction. Draw curved arrows to show the electron flow that has occurred in each step.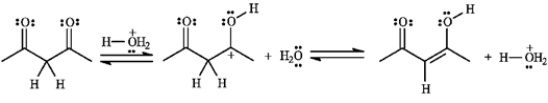
The structures below show the stepwise bond making and bond breaking in this reaction. Draw curved arrows to show the electron flow that has occurred in each step.


Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
22
Use the second and third steps of the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following question(s). 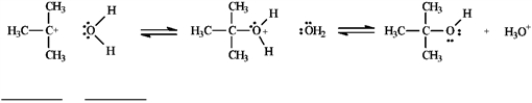
Refer to instructions. Draw arrows on the structures above showing electron flow in steps two and three of this reaction.

Refer to instructions. Draw arrows on the structures above showing electron flow in steps two and three of this reaction.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
23
Add curved arrows to the following reaction(s) to indicate the flow of electrons in each.
Indicate flow: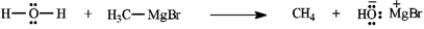
Indicate flow:
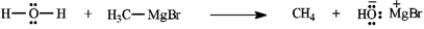

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
24
Use the reaction energy diagram below to answer the following question(s). 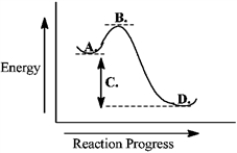
The transition state is found at _____ on the diagram.
A)A
B)B
C)C
D)D

The transition state is found at _____ on the diagram.
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
25
In the reaction below:
a)Label the nucleophile (Nu) and the electrophile (E).
b)Draw arrows on the structures showing electron flow in the reaction.
Label and indicate flow: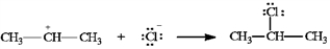
a)Label the nucleophile (Nu) and the electrophile (E).
b)Draw arrows on the structures showing electron flow in the reaction.
Label and indicate flow:
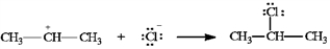

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
26
Match each definition to one of the terms below.
The energy needed by reactants to reach the transition state.
A)transition state
B)endergonic reaction
C)activation energy
D)Gibbs free energy change
E)exergonic reaction
F)reaction intermediate
The energy needed by reactants to reach the transition state.
A)transition state
B)endergonic reaction
C)activation energy
D)Gibbs free energy change
E)exergonic reaction
F)reaction intermediate

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
27
Match each definition to one of the terms below.
Another term used for a Lewis acid.
A)polarization
B)addition reaction
C)radical reaction
D)electrophile
E)polar reaction
F)substitution
G)nucleophile
H)elimination reaction
Another term used for a Lewis acid.
A)polarization
B)addition reaction
C)radical reaction
D)electrophile
E)polar reaction
F)substitution
G)nucleophile
H)elimination reaction

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
28
Use the reaction energy diagram below to answer the following question(s). 
The free-energy change for the reaction is indicated at _____ on the diagram.
A)A
B)B
C)C
D)D

The free-energy change for the reaction is indicated at _____ on the diagram.
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
29
Match each definition to one of the terms below.
ΔG° = −RT ln Keq
A)transition state
B)endergonic reaction
C)activation energy
D)Gibbs free energy change
E)exergonic reaction
F)reaction intermediate
ΔG° = −RT ln Keq
A)transition state
B)endergonic reaction
C)activation energy
D)Gibbs free energy change
E)exergonic reaction
F)reaction intermediate

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
30
Match each definition to one of the terms below.
A reaction where ΔG° is positive.
A)transition state
B)endergonic reaction
C)activation energy
D)Gibbs free energy change
E)exergonic reaction
F)reaction intermediate
A reaction where ΔG° is positive.
A)transition state
B)endergonic reaction
C)activation energy
D)Gibbs free energy change
E)exergonic reaction
F)reaction intermediate

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
31
Add curved arrows to the following reaction(s) to indicate the flow of electrons in each.
If the yield for the following reaction is 76%, calculate Keq and predict the sign of ΔG°.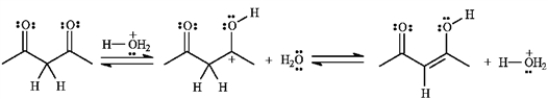
If the yield for the following reaction is 76%, calculate Keq and predict the sign of ΔG°.


Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
32
Match each definition to one of the terms below.
A reaction that involves a species with an unpaired electron.
A)polarization
B)addition reaction
C)radical reaction
D)electrophile
E)polar reaction
F)substitution
G)nucleophile
H)elimination reaction
A reaction that involves a species with an unpaired electron.
A)polarization
B)addition reaction
C)radical reaction
D)electrophile
E)polar reaction
F)substitution
G)nucleophile
H)elimination reaction

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
33
In the reaction below:
a)Label the nucleophile (Nu) and the electrophile (E).
b)Draw arrows on the structures showing electron flow in the reaction.
Predict the two alcohol addition products obtained by reaction of the following alkene with aqueous acid.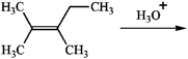
a)Label the nucleophile (Nu) and the electrophile (E).
b)Draw arrows on the structures showing electron flow in the reaction.
Predict the two alcohol addition products obtained by reaction of the following alkene with aqueous acid.


Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
34
In the reaction below:
a)Label the nucleophile (Nu) and the electrophile (E).
b)Draw arrows on the structures showing electron flow in the reaction.
Predict the product of the following reaction of Prostaglandin H2 by interpreting the flow of electrons as indicated by the curved arrows.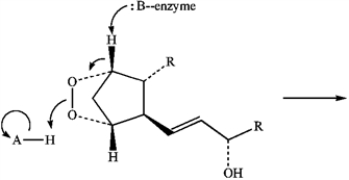
a)Label the nucleophile (Nu) and the electrophile (E).
b)Draw arrows on the structures showing electron flow in the reaction.
Predict the product of the following reaction of Prostaglandin H2 by interpreting the flow of electrons as indicated by the curved arrows.


Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
35
Match each definition to one of the terms below.
A species that lies at an energy maximum during an individual step in a reaction.
A)transition state
B)endergonic reaction
C)activation energy
D)Gibbs free energy change
E)exergonic reaction
F)reaction intermediate
A species that lies at an energy maximum during an individual step in a reaction.
A)transition state
B)endergonic reaction
C)activation energy
D)Gibbs free energy change
E)exergonic reaction
F)reaction intermediate

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
36
Use the reaction energy diagram below to answer the following question(s). 
The products are found at _____ on the diagram.
A)A
B)B
C)C
D)D

The products are found at _____ on the diagram.
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
37
Add curved arrows to the following reaction(s) to indicate the flow of electrons in each.
Consider the following reaction: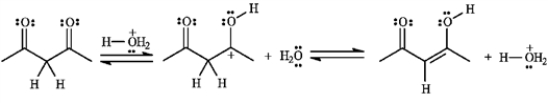 a)Assuming that the first step is the slow step, draw and label a qualitative energy diagram for the reaction.
a)Assuming that the first step is the slow step, draw and label a qualitative energy diagram for the reaction.
b)If the second step of the reaction were the slow step, briefly explain how the values of ΔG‡1, ΔG‡2, and ΔG° would change.
Consider the following reaction:
 a)Assuming that the first step is the slow step, draw and label a qualitative energy diagram for the reaction.
a)Assuming that the first step is the slow step, draw and label a qualitative energy diagram for the reaction.b)If the second step of the reaction were the slow step, briefly explain how the values of ΔG‡1, ΔG‡2, and ΔG° would change.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
38
Use the second and third steps of the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following question(s). 
Refer to instructions. Label the nucleophile, Nu, and the electrophile, E+, in the blanks provided under the structures.

Refer to instructions. Label the nucleophile, Nu, and the electrophile, E+, in the blanks provided under the structures.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
39
Use the reaction energy diagram below to answer the following question(s). 
The reactants are found at _____ on the diagram.
A)A
B)B
C)C
D)D

The reactants are found at _____ on the diagram.
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck


