Deck 2: Polar Covalent Bonds; Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/41
Play
Full screen (f)
Deck 2: Polar Covalent Bonds; Acids and Bases
1
Phenylalanine is an amino acid that is essential to human nutrition. The representation below shows the structure of phenylalanine at the pH found in cells. Consider this structure to answer the following question(s). 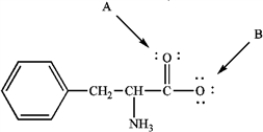 phenylalanine
phenylalanine
Refer to instructions. Assign any formal charges to atoms in this representation of phenylalanine.
 phenylalanine
phenylalanineRefer to instructions. Assign any formal charges to atoms in this representation of phenylalanine.
2
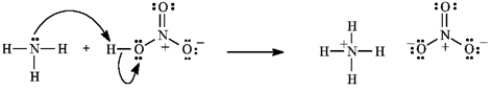
Consider the reaction below to answer the following question. 
Refer to instructions. Using the curved arrow formalism, show the flow of electrons for this reaction.

Refer to instructions. Using the curved arrow formalism, show the flow of electrons for this reaction.
3
What is the formal charge on the nitrogen atom indicated with the arrow in the following compound? 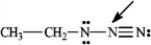
A) 0
B) −1
C) +1
D) −2
E) +2
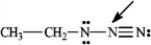
A) 0
B) −1
C) +1
D) −2
E) +2
+1
4
Use the convention δ−/δ+ and the crossed arrow (  ) to show the direction of the expected polarity of the indicated bonds in the following compound(s).
) to show the direction of the expected polarity of the indicated bonds in the following compound(s).
Refer to instructions. A C−O bond in tetrahydrofuran,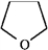
 ) to show the direction of the expected polarity of the indicated bonds in the following compound(s).
) to show the direction of the expected polarity of the indicated bonds in the following compound(s).Refer to instructions. A C−O bond in tetrahydrofuran,


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
5
Phenylalanine is an amino acid that is essential to human nutrition. The representation below shows the structure of phenylalanine at the pH found in cells. Consider this structure to answer the following question(s).  phenylalanine
phenylalanine
Refer to instructions. The oxygen atom labeled "B" has _____ nonbonding electrons.
 phenylalanine
phenylalanineRefer to instructions. The oxygen atom labeled "B" has _____ nonbonding electrons.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
6
Refer to the following equation to answer the question(s) below. 
Refer to instructions. The strongest Brønsted-Lowry acid in the equation is indicated by letter _____.
A)A
B)B
C)C
D)D

Refer to instructions. The strongest Brønsted-Lowry acid in the equation is indicated by letter _____.
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
7
Among the following compounds which can function only as a Brønsted-Lowry base? A 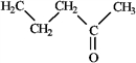 B
B 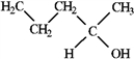 C
C 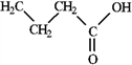 D
D 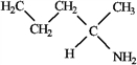
A) A
B) B
C) C
D) D
 B
B  C
C  D
D 
A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
8
Use the convention δ−/δ+ and the crossed arrow (  ) to show the direction of the expected polarity of the indicated bonds in the following compound(s).
) to show the direction of the expected polarity of the indicated bonds in the following compound(s).
Refer to instructions. The C−F bond in fluorobenzene,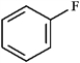
 ) to show the direction of the expected polarity of the indicated bonds in the following compound(s).
) to show the direction of the expected polarity of the indicated bonds in the following compound(s).Refer to instructions. The C−F bond in fluorobenzene,


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
9
Consider the species below to answer the following question.BF3
Fe2+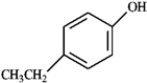
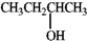
Refer to instructions. Which of the following would be common to all?
A) Lewis acids
B) Lewis bases
C) Lewis acids or bases
D) Neither Lewis acids nor bases
Fe2+

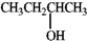
Refer to instructions. Which of the following would be common to all?
A) Lewis acids
B) Lewis bases
C) Lewis acids or bases
D) Neither Lewis acids nor bases

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following molecules would exhibit the largest dipole moment?
A) CH3CH3
B) CH3CH2F
C) CH3CH2Cl
D) CH3CH2Br
A) CH3CH3
B) CH3CH2F
C) CH3CH2Cl
D) CH3CH2Br

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
11
Draw two resonance structures for the species below. 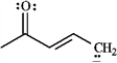


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
12
The following shows an intermediate used in a Grignard synthesis. Which atom will inductively donate electrons in this species? 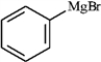
A) C
B) Br
C) Mg
D) H
E) both b and c

A) C
B) Br
C) Mg
D) H
E) both b and c

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
13
Label the acid and base in each reaction below.
Label:
Label:


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
14
Indole is pleasant smelling in highly dilute solutions and has been used in perfumery. Use the structure of indole, below, to answer the following question(s). 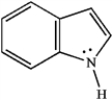
Refer to instructions. Indole can function as a Brønsted-Lowry acid in the presence of strong bases. Formulate a reaction, using a generic base (:B−), showing electron flow with arrows, that demonstrates this reactivity of indole.

Refer to instructions. Indole can function as a Brønsted-Lowry acid in the presence of strong bases. Formulate a reaction, using a generic base (:B−), showing electron flow with arrows, that demonstrates this reactivity of indole.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
15
Refer to the following equation to answer the question(s) below. 
Refer to instructions. The strongest Brønsted-Lowry base in the equation is indicated by letter _____.
A)A
B)B
C)C
D)D

Refer to instructions. The strongest Brønsted-Lowry base in the equation is indicated by letter _____.
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
16
Refer to the following equation to answer the question(s) below. 
Refer to instructions. Will this reaction take place as written in the forward direction? Explain.

Refer to instructions. Will this reaction take place as written in the forward direction? Explain.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
17
Phenylalanine is an amino acid that is essential to human nutrition. The representation below shows the structure of phenylalanine at the pH found in cells. Consider this structure to answer the following question(s).  phenylalanine
phenylalanine
Refer to instructions. The oxygen atom labeled "A" has ______ bonding electron pairs.
 phenylalanine
phenylalanineRefer to instructions. The oxygen atom labeled "A" has ______ bonding electron pairs.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
18
Consider the structure of acetic acid shown below. 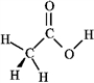 In the electrostatic potential map of acetic acid, in which of the following bonds would the terminal atom appear as the deepest shade of red?
In the electrostatic potential map of acetic acid, in which of the following bonds would the terminal atom appear as the deepest shade of red?
A) C=O
B) C−H
C) C−C
D) O−H
 In the electrostatic potential map of acetic acid, in which of the following bonds would the terminal atom appear as the deepest shade of red?
In the electrostatic potential map of acetic acid, in which of the following bonds would the terminal atom appear as the deepest shade of red?A) C=O
B) C−H
C) C−C
D) O−H

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
19
Circle the Lewis bases in the group of compounds below. 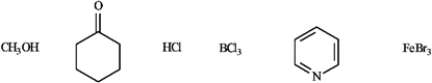


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
20
Explain why phenol has a lower pKa than ethanol. 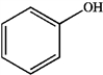
 phenol
phenol
ethanol

 phenol
phenolethanol

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
21
Write an equation for the reaction of the alkaloid ephedrine with a proton, showing the structure of its conjugate base. 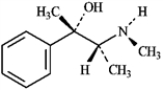


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
22
The visual pigment in animal cells consists of an isomer of retinal linked to the protein opsin, as shown below. Write an alternative resonance form for this species that shows the positive charge situated on the starred carbon instead of the nitrogen atom and include the curved arrows to show the electron flow. 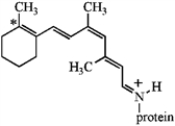


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
23
Guanidine is a fairly strong amine base that is attached to the amino acid arginine. Draw three resonance forms for the conjugate acid of guanidine. 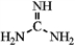 guanidine
guanidine
 guanidine
guanidine
Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following statements is not true regarding resonance structures?
A) All resonance structures must have the same number of electrons
B) All resonance structures must differ in the hybridization of atoms.
C) All resonance structures must have the same arrangement of atoms
D) All resonance structures must be valid Lewis structures
A) All resonance structures must have the same number of electrons
B) All resonance structures must differ in the hybridization of atoms.
C) All resonance structures must have the same arrangement of atoms
D) All resonance structures must be valid Lewis structures

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
25
In aromatic nitration reactions, nitric acid (HNO3) is used in conjunction with the stronger acid, sulfuric acid, H2SO4, to form an intermediate. Which of the following could be the formula for this intermediate?
A) NO3−
B) H3SO4+
C) H2NO3+
D) HNO2
A) NO3−
B) H3SO4+
C) H2NO3+
D) HNO2

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
26
Draw the conjugate base of each species. 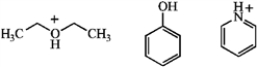


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
27
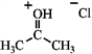
Identify the reactants and product in the reaction below as acids or bases and specify whether they are Lewis and/or Brønsted-Lowry. 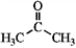





Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
28
Write an equation for the reaction of boron trifluoride, an important reagent in organic chemistry, with trimethylamine. Represent the movement of electrons with a curved arrow, and show the formal charges on the atoms in the product. 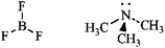
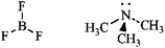

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
29
The condensed structure for dimethyl ether looks symmetrical. However, dimethyl ether has a dipole moment. Draw a structure that explains this and indicate the expected direction of the molecular dipole moment. 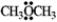 dimethyl ether
dimethyl ether
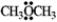 dimethyl ether
dimethyl ether
Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
30
Use the curved arrow method to show the electron movement, and label the acid, base, conjugate acid, and conjugate base. 


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
31
Rank the following in order of decreasing importance as a contributing resonance structure to the molecular structure of acetone, CH3COCH3 (more important > less important). A 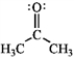 B
B 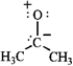 C
C 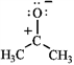
A) A > B > C
B) A > C > B
C) B > A > C
D) C > A > B
 B
B  C
C 
A) A > B > C
B) A > C > B
C) B > A > C
D) C > A > B

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
32
Which is the strongest base (pKa values given for conjugate acid)?
A) NH3 (pKa = 9.2)
B) CH3O− (pKa = 16)
C)
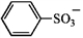 (pKa = −6.5)
(pKa = −6.5)
D) CH3CO2− (pKa = 4.7)
E) H− (pKa = 35)
A) NH3 (pKa = 9.2)
B) CH3O− (pKa = 16)
C)
 (pKa = −6.5)
(pKa = −6.5)D) CH3CO2− (pKa = 4.7)
E) H− (pKa = 35)

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
33
Consider the molecules below to answer the following question.CH3CH2NH2
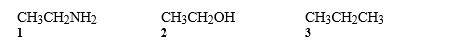
Refer to instructions. Which of the following is an accurate description of the noncovalent interactions between like molecules?
A) Only 1 exhibits hydrogen bonding.
B) Only 2 exhibits hydrogen bonding.
C) Only 3 exhibits hydrogen bonding.
D) Only 1 and 2 exhibit hydrogen bonding.
E) All of these exhibit hydrogen bonding.
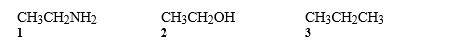
Refer to instructions. Which of the following is an accurate description of the noncovalent interactions between like molecules?
A) Only 1 exhibits hydrogen bonding.
B) Only 2 exhibits hydrogen bonding.
C) Only 3 exhibits hydrogen bonding.
D) Only 1 and 2 exhibit hydrogen bonding.
E) All of these exhibit hydrogen bonding.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
34
Complete this acid-base reaction, and label the acid, base, conjugate base, and conjugate acid. 


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
35
Draw two resonance forms for the cyclopentadienyl radical. 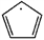


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
36
Draw a Lewis structure for each of the following.
a)hydroxylammonium ion: +NH3OH.
b)azide ion: (N3−)
a)hydroxylammonium ion: +NH3OH.
b)azide ion: (N3−)

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
37
Indole is pleasant smelling in highly dilute solutions and has been used in perfumery. Use the structure of indole, below, to answer the following question(s). 
Refer to instructions. Indole can function as a Lewis base in the presence of strong acid. Formulate a reaction, using a generic acid (HA), showing electron flow with arrows, that demonstrates this reactivity of indole.

Refer to instructions. Indole can function as a Lewis base in the presence of strong acid. Formulate a reaction, using a generic acid (HA), showing electron flow with arrows, that demonstrates this reactivity of indole.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following molecules has a dipole moment?
A)
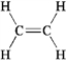
B)
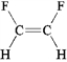
C)
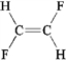
D)
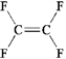
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
39
Of the bonds found in 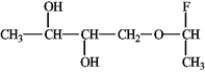 which is the most polar?
which is the most polar?
A) C−F
B) O−H
C) C−H
D) C−O
 which is the most polar?
which is the most polar?A) C−F
B) O−H
C) C−H
D) C−O

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
40
Consider the molecules below to answer the following question.CH3CH2NH2
The following is the structure of vitamin C. This compound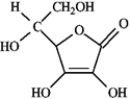
A) is hydrophilic.
B) exhibits dispersion forces.
C) exhibits hydrogen bonding.
D) would be soluble in water.
E) all of these.
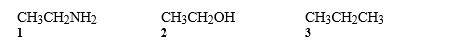
The following is the structure of vitamin C. This compound

A) is hydrophilic.
B) exhibits dispersion forces.
C) exhibits hydrogen bonding.
D) would be soluble in water.
E) all of these.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
41
Consider the molecules below to answer the following question.CH3CH2NH2
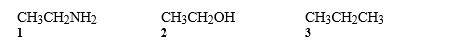
Which of the following exhibits only dispersion forces? A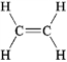 B
B 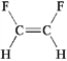 C
C 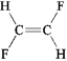 D
D 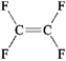
A) only A
B) only B
C) only C
D) only D
E) all except B
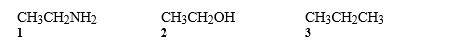
Which of the following exhibits only dispersion forces? A
 B
B  C
C  D
D 
A) only A
B) only B
C) only C
D) only D
E) all except B

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck


