Deck 21: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/44
Play
Full screen (f)
Deck 21: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions
1
Exhibit 21-2
Consider the reaction below to answer the following question(s): Acid halides react with diazomethane to yield diazoketones.Excess diazomethane is used to prevent the HCl produced in the reaction from reacting with the diazoketone.
Acid halides react with diazomethane to yield diazoketones.Excess diazomethane is used to prevent the HCl produced in the reaction from reacting with the diazoketone.
Refer to Exhibit 21-2.Diazomethane is an example of a dipolar molecule;a molecule which is neutral overall but has charges on individual atoms.One resonance form of diazomethane is drawn below.Draw the Lewis structure of the other resonance form of diazomethane.Be sure to include all formal charges.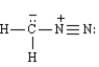
Consider the reaction below to answer the following question(s):
 Acid halides react with diazomethane to yield diazoketones.Excess diazomethane is used to prevent the HCl produced in the reaction from reacting with the diazoketone.
Acid halides react with diazomethane to yield diazoketones.Excess diazomethane is used to prevent the HCl produced in the reaction from reacting with the diazoketone.Refer to Exhibit 21-2.Diazomethane is an example of a dipolar molecule;a molecule which is neutral overall but has charges on individual atoms.One resonance form of diazomethane is drawn below.Draw the Lewis structure of the other resonance form of diazomethane.Be sure to include all formal charges.
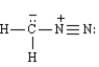

2
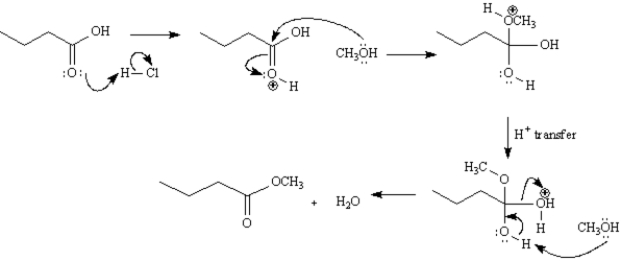
Methyl butanoate has been isolated from pineapple oil and can be prepared by the Fischer esterification reaction shown below.Write the complete stepwise mechanism for this reaction.Show all electron flow with arrows and include all intermediate structures. 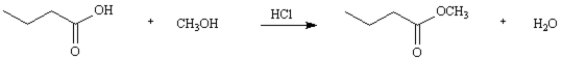

3
Draw: (E)-2,4-dimethyl-2-hexenoyl chloride
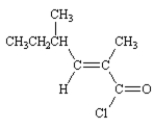
4
Draw: N,N-dimethylformamide

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
5
Exhibit 21-3
Consider the reaction below to answer the following question(s):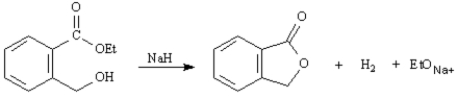
Refer to Exhibit 21-3.The purpose of the base catalyst in this reaction is:
A)to polarize the carbonyl group to make it more electrophilic
B)to convert the ester to an intermediate carboxylic acid
C)to convert the alcohol group to an alkoxide anion,which is a better nucleophile
D)all of the above
Consider the reaction below to answer the following question(s):

Refer to Exhibit 21-3.The purpose of the base catalyst in this reaction is:
A)to polarize the carbonyl group to make it more electrophilic
B)to convert the ester to an intermediate carboxylic acid
C)to convert the alcohol group to an alkoxide anion,which is a better nucleophile
D)all of the above

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
6
Draw: acetic formic anhydride

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
7
Name: 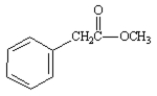


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
8
Draw: 2-propenamide

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
9
Draw: methyl cis-3-ethylcyclobutanecarboxylate

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
10
Name: 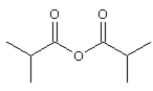


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
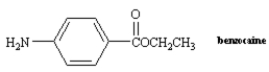
11
Name:


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
12
Name: 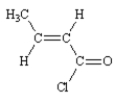


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
13
Write the complete stepwise mechanism for the acid-catalyzed hydrolysis of the following amide to yield mandelic acid.Show all electron flow with arrows and draw the structures of all intermediate species. 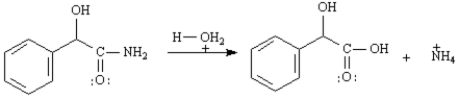


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
14
Exhibit 21-3
Consider the reaction below to answer the following question(s):
Refer to Exhibit 21-3.Write the complete stepwise mechanism for this reaction.Show intermediate structures and all electron flow with arrows.
Consider the reaction below to answer the following question(s):

Refer to Exhibit 21-3.Write the complete stepwise mechanism for this reaction.Show intermediate structures and all electron flow with arrows.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
15
Draw: 3,4,5-trimethoxybenzoyl chloride

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
16
Exhibit 21-2
Consider the reaction below to answer the following question(s): Acid halides react with diazomethane to yield diazoketones.Excess diazomethane is used to prevent the HCl produced in the reaction from reacting with the diazoketone.
Acid halides react with diazomethane to yield diazoketones.Excess diazomethane is used to prevent the HCl produced in the reaction from reacting with the diazoketone.
Refer to Exhibit 21-2.The intermediate structures for the mechanism for the reaction of propanyl chloride with diazomethane are provide below.Show all electron flow with arrows on these structures.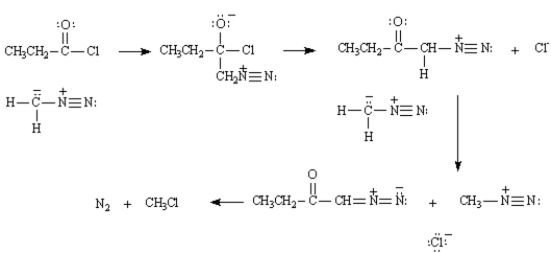
Consider the reaction below to answer the following question(s):
 Acid halides react with diazomethane to yield diazoketones.Excess diazomethane is used to prevent the HCl produced in the reaction from reacting with the diazoketone.
Acid halides react with diazomethane to yield diazoketones.Excess diazomethane is used to prevent the HCl produced in the reaction from reacting with the diazoketone.Refer to Exhibit 21-2.The intermediate structures for the mechanism for the reaction of propanyl chloride with diazomethane are provide below.Show all electron flow with arrows on these structures.


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
17
Exhibit 21-3
Consider the reaction below to answer the following question(s):
Refer to Exhibit 21-3.This reaction is an example of:
A)an intermolecular nucleophilic acyl substitution reaction
B)an intramolecular nucleophilic acyl substitution reaction
C)an intermolecular SN2 reaction
D)an intramolecular SN2 reaction
Consider the reaction below to answer the following question(s):

Refer to Exhibit 21-3.This reaction is an example of:
A)an intermolecular nucleophilic acyl substitution reaction
B)an intramolecular nucleophilic acyl substitution reaction
C)an intermolecular SN2 reaction
D)an intramolecular SN2 reaction

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
18
Name: 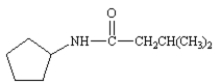


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
19
Exhibit 21-3
Consider the reaction below to answer the following question(s):
Refer to Exhibit 21-3.The product of this reaction is:
A)a lactone
B)an anhydride
C)a lactam
D)an ether
Consider the reaction below to answer the following question(s):

Refer to Exhibit 21-3.The product of this reaction is:
A)a lactone
B)an anhydride
C)a lactam
D)an ether

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
20
What is the correct structure for phenylbenzoate?
A)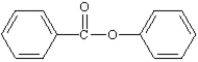
B)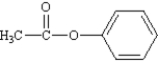
C)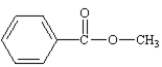
D)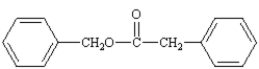
A)

B)

C)

D)
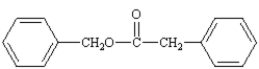

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
21
Name the following substance.Atoms other than carbon and hydrogen are labeled. 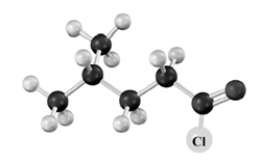


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
22
Which line in the following spectrum would indicate the presence of a carbonyl group? 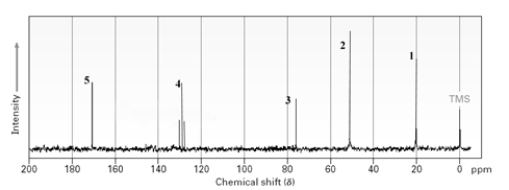
A)1
B)2
C)3
D)4
E)5

A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
23
Ethyl phenylacetate is a pleasant smelling compound used in perfumery.Draw structures for each of the intermediates in the synthesis of ethyl phenylacetate below. 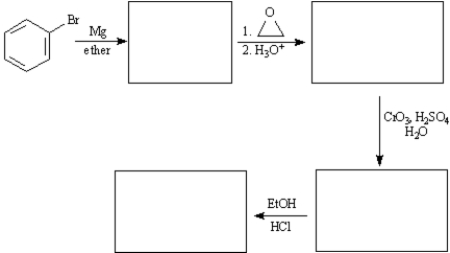


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
24
Exhibit 21-4
Consider the information below to answer the following question(s).
The reaction of a carboxylic acid with an alcohol in the presence of acid is termed Fischer esterification.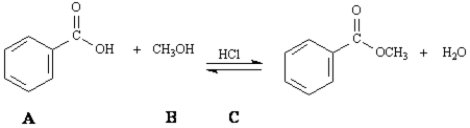
Refer to Exhibit 21-4.Write the stepwise mechanism for the Fischer esterification reaction of benzoic acid and methanol given above.Show all electron flow by using curved arrows,and include all intermediate structures.
Consider the information below to answer the following question(s).
The reaction of a carboxylic acid with an alcohol in the presence of acid is termed Fischer esterification.

Refer to Exhibit 21-4.Write the stepwise mechanism for the Fischer esterification reaction of benzoic acid and methanol given above.Show all electron flow by using curved arrows,and include all intermediate structures.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
25
Consider the following molecular model.Atoms other than carbon and hydrogen are labeled. 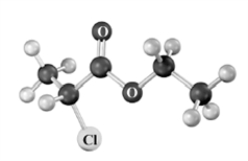 Which of the following reactants could be used to produce this compound?
Which of the following reactants could be used to produce this compound?
A)2-chloropropanoic acid and ethanol
B)propanoic acid and chloroethanol
C)propanoic acid and ethanol
 Which of the following reactants could be used to produce this compound?
Which of the following reactants could be used to produce this compound?A)2-chloropropanoic acid and ethanol
B)propanoic acid and chloroethanol
C)propanoic acid and ethanol

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
26
What is the name of the product when the following substance reacts with NaCH3COO? Atoms other than carbon and hydrogen are labeled. 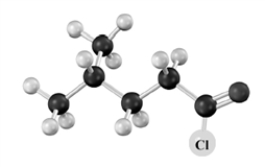
A)4-methylbutanoic acid
B)acetic 4-methylbutanoic anhydride
C)4-methylbutyl acetate
D)acetyl 4-methylbutanoate
E)No reaction occurs.

A)4-methylbutanoic acid
B)acetic 4-methylbutanoic anhydride
C)4-methylbutyl acetate
D)acetyl 4-methylbutanoate
E)No reaction occurs.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
27
Aklomide,2-chloro-4-nitrobenzamide,is an ingredient in veterinary antibacterial preparations.Propose a synthesis of aklomide starting with toluene.Show all reagents and all intermediate structures. 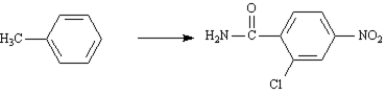


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
28
Based on the following spectrum 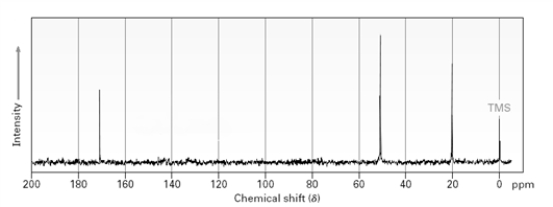 the sample used would probably be classified as a(n):
the sample used would probably be classified as a(n):
A)aldehyde.
B)ketone.
C)ester.
D)The spectrum is not specific enough to classify the substance.
 the sample used would probably be classified as a(n):
the sample used would probably be classified as a(n):A)aldehyde.
B)ketone.
C)ester.
D)The spectrum is not specific enough to classify the substance.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following will take place via nucleophilic acyl substitution?
A)thioester producing an acid chloride
B)ester producing a thioester
C)ester producing an amide
D)acid anhydride producing an acid chloride
A)thioester producing an acid chloride
B)ester producing a thioester
C)ester producing an amide
D)acid anhydride producing an acid chloride

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
30
The purpose of the acid catalyst in the hydrolysis of an amide is:
A)to enhance the nucleophilicity of the water molecule
B)to enhance the electrophilicity of the amide carbonyl carbon
C)to enhance the electrophilicity of the water molecule
D)to shift the equilibrium of the reaction
A)to enhance the nucleophilicity of the water molecule
B)to enhance the electrophilicity of the amide carbonyl carbon
C)to enhance the electrophilicity of the water molecule
D)to shift the equilibrium of the reaction

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
31
Name the following substance.Atoms other than carbon and hydrogen are labeled. 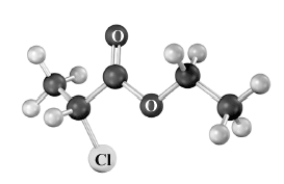


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
32
The following is an excerpt from a spectrum taken on an unknown sample. 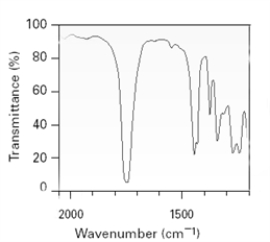 To which of the following functional groups might the unknown belong?
To which of the following functional groups might the unknown belong?
A)amide
B)acid chloride
C)carboxylic acid
D)ester
E)None of these can be eliminated.
 To which of the following functional groups might the unknown belong?
To which of the following functional groups might the unknown belong?A)amide
B)acid chloride
C)carboxylic acid
D)ester
E)None of these can be eliminated.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
33
Exhibit 21-4
Consider the information below to answer the following question(s).
The reaction of a carboxylic acid with an alcohol in the presence of acid is termed Fischer esterification.
Refer to Exhibit 21-4.Fischer esterification is an example of:
A)nucleophilic acyl addition
B)nucleophilic acyl substitution
C)nucleophilic acyl elimination
D)nucleophilic acyl rearrangement
Consider the information below to answer the following question(s).
The reaction of a carboxylic acid with an alcohol in the presence of acid is termed Fischer esterification.

Refer to Exhibit 21-4.Fischer esterification is an example of:
A)nucleophilic acyl addition
B)nucleophilic acyl substitution
C)nucleophilic acyl elimination
D)nucleophilic acyl rearrangement

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
34
Poly(ethylene terephthalate),PET,is the polymeric material of Mylar and Dacron .What are the monomers from which PET is prepared? 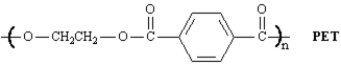


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
35
Exhibit 21-9
Refer to the data below to answer the following question(s):
Kodel is a staple and filament fiber prepared from dimethyl terephthalate and 1,4-cyclohexanedimethanol.Fabric made from Kodel has good crease resistance.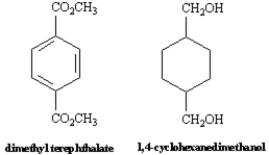
Refer to Exhibit 21-9.Draw the structure of the Kodel polymer.
Refer to the data below to answer the following question(s):
Kodel is a staple and filament fiber prepared from dimethyl terephthalate and 1,4-cyclohexanedimethanol.Fabric made from Kodel has good crease resistance.

Refer to Exhibit 21-9.Draw the structure of the Kodel polymer.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
36
Consider the structure given below which might be found in a protein. 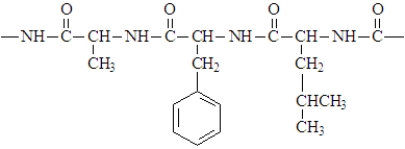 Which of the following is not applicable to this structure?
Which of the following is not applicable to this structure?
A)contains three monomers
B)contains amide bonds
C)formed from a diacid and diamine
D)contains three types of monomers
E)All of these apply to this structure.
 Which of the following is not applicable to this structure?
Which of the following is not applicable to this structure?A)contains three monomers
B)contains amide bonds
C)formed from a diacid and diamine
D)contains three types of monomers
E)All of these apply to this structure.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
37
Exhibit 21-4
Consider the information below to answer the following question(s).
The reaction of a carboxylic acid with an alcohol in the presence of acid is termed Fischer esterification.
Refer to Exhibit 21-4.Compound C functions as _____ in this reaction.
A)a base scavenger
B)a solvent
C)a catalyst
D)a neutralizer
Consider the information below to answer the following question(s).
The reaction of a carboxylic acid with an alcohol in the presence of acid is termed Fischer esterification.

Refer to Exhibit 21-4.Compound C functions as _____ in this reaction.
A)a base scavenger
B)a solvent
C)a catalyst
D)a neutralizer

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
38
What is the product when the following substance reacts with trimethylamine ((CH3)3N)? Atoms other than carbon and hydrogen are labeled. 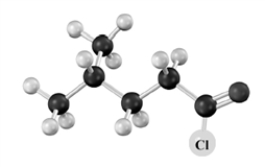
A)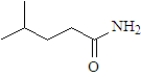
B)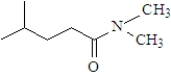
C)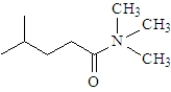
D)No reaction occurs.

A)

B)

C)

D)No reaction occurs.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
39
Exhibit 21-9
Refer to the data below to answer the following question(s):
Kodel is a staple and filament fiber prepared from dimethyl terephthalate and 1,4-cyclohexanedimethanol.Fabric made from Kodel has good crease resistance.
Refer to Exhibit 21-9.Kodel is an example of:
A)a polyurethane
B)a polyester
C)a polyamide
D)a polycarbonate
Refer to the data below to answer the following question(s):
Kodel is a staple and filament fiber prepared from dimethyl terephthalate and 1,4-cyclohexanedimethanol.Fabric made from Kodel has good crease resistance.

Refer to Exhibit 21-9.Kodel is an example of:
A)a polyurethane
B)a polyester
C)a polyamide
D)a polycarbonate

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
40
Exhibit 21-4
Consider the information below to answer the following question(s).
The reaction of a carboxylic acid with an alcohol in the presence of acid is termed Fischer esterification.
Refer to Exhibit 21-4.The nucleophile in this reaction is _____.
Consider the information below to answer the following question(s).
The reaction of a carboxylic acid with an alcohol in the presence of acid is termed Fischer esterification.

Refer to Exhibit 21-4.The nucleophile in this reaction is _____.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
41
Draw the mechanism of acid-catalyzed hydrolysis of propyl ethanoate to yield propanoic acid.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
42
Draw the mechanism of base-induced hydrolysis of ethyl propanoate.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following describes nucleophilic acyl substitution?
A)Nucleophile adds to the electrophilic carbon of the carbonyl bond.
B)Electrophile replaces a group on the carbon alpha to the carbonyl.
C)Nucleophile replaces a group adjacent to the carbon of carbonyl.
D)Two carbonyl groups react with each other.
A)Nucleophile adds to the electrophilic carbon of the carbonyl bond.
B)Electrophile replaces a group on the carbon alpha to the carbonyl.
C)Nucleophile replaces a group adjacent to the carbon of carbonyl.
D)Two carbonyl groups react with each other.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
44
Draw the mechanism of the Fischer esterification of butanoic acid,in the presence of ethanol and hydrochloric acid,which is the catalyst.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck


