
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Edition 4ISBN: 978-0138004644
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Edition 4ISBN: 978-0138004644 Exercise 3
The decomposition of crystalline N2 O5 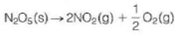
is an example of a reaction that is thermodynamically favored even though it absorbs heat. At 25 °C we have the following values for the standard state enthalpy and free energy changes of the reaction: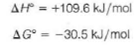
(a) Calculate S ° at 25 °C.
(b) Why is the entropy change so favorable for this reaction
(c) Calculate U° for this reaction at 25 °C.
(d) Why is H ° greater than U °
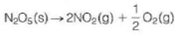
is an example of a reaction that is thermodynamically favored even though it absorbs heat. At 25 °C we have the following values for the standard state enthalpy and free energy changes of the reaction:
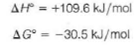
(a) Calculate S ° at 25 °C.
(b) Why is the entropy change so favorable for this reaction
(c) Calculate U° for this reaction at 25 °C.
(d) Why is H ° greater than U °
Explanation
The data provided for decomposition of c...
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


