
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Edition 4ISBN: 978-0138004644
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Edition 4ISBN: 978-0138004644 Exercise 5
The first reaction in glycolysis is the phosphorylation of glucose: 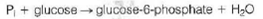
This is a thermodynamically unfavorable process, with G° = +13.8 kJ/mol.
(a) In a liver cell at 37 °C the concentrations of both phosphate and glucose are normally maintained at about 5 mM each. What would be the equilibrium concentration of glucose-6-phosphate, according to the above data
(b) This very low concentration of the desired product would be unfavorable for glycolysis. In fact, the reaction is coupled to ATP hydrolysis to give the overall reaction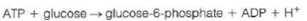
What is G° for the coupled reaction
(c) If, in addition to the constraints on glucose concentration fisted previously, we have in the liver cell ATP concentration = 3 mM and ADP concentration = 1 mM, what is the theoretical concentration of glucose-6-phosphate at equilibrium at pH = 7.4 and 37 °C The answer you will obtain is an absurdly high value for the cell and in fact is never approached in reality. Explain why.
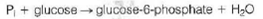
This is a thermodynamically unfavorable process, with G° = +13.8 kJ/mol.
(a) In a liver cell at 37 °C the concentrations of both phosphate and glucose are normally maintained at about 5 mM each. What would be the equilibrium concentration of glucose-6-phosphate, according to the above data
(b) This very low concentration of the desired product would be unfavorable for glycolysis. In fact, the reaction is coupled to ATP hydrolysis to give the overall reaction
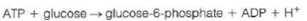
What is G° for the coupled reaction
(c) If, in addition to the constraints on glucose concentration fisted previously, we have in the liver cell ATP concentration = 3 mM and ADP concentration = 1 mM, what is the theoretical concentration of glucose-6-phosphate at equilibrium at pH = 7.4 and 37 °C The answer you will obtain is an absurdly high value for the cell and in fact is never approached in reality. Explain why.
Explanation
For the phosphorylation of glucose to gl...
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


