
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Edition 4ISBN: 978-0138004644
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Edition 4ISBN: 978-0138004644 Exercise 6
In another key reaction in glycolysis, dihydroxyacetone phosphate (DHAP) is isomerized into glyceraldehyde-3-phosphate (GAP): 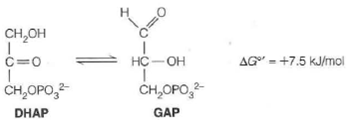
Because G° is positive, the equilibrium lies to the left.
(a) Calculate the equilibrium constant, and the equilibrium fraction of GAP from the above, at 37 °C.
(b) In the cell, depletion of GAP makes the reaction proceed. What will AG be if the concentration of GAP is always kept at 1/100 of the concentration of DHAP

Because G° is positive, the equilibrium lies to the left.
(a) Calculate the equilibrium constant, and the equilibrium fraction of GAP from the above, at 37 °C.
(b) In the cell, depletion of GAP makes the reaction proceed. What will AG be if the concentration of GAP is always kept at 1/100 of the concentration of DHAP
Explanation
In glycolysis pathway two important chem...
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


