
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Edition 4ISBN: 978-0138004644
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Edition 4ISBN: 978-0138004644 Exercise 10
Suppose a reaction has H ° and S ° values independent of temperature. Show from this, and equations given in this chapter, that 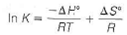
where K is the equilibrium constant. How could you use values of K determined at different temperatures to determine H ° for the reaction
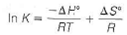
where K is the equilibrium constant. How could you use values of K determined at different temperatures to determine H ° for the reaction
Explanation
Gibb's free energy is a state function a...
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


