
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Edition 4ISBN: 978-0138004644
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Edition 4ISBN: 978-0138004644 Exercise 12
The phosphate transfer potentials for glucose-1-phosphate and glucose-6-phosphate are 20.9 kJ/mol and 13.8 kJ/mol, respectively.
(a) What is the equilibrium constant for this reaction at 25 °C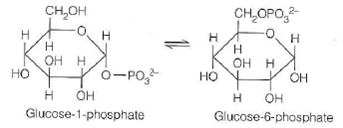
(b) If a mixture was prepared containing 1 M glucose-6-phosphate and 1 × 10 3 M glucose-1-phosphate, what would be the thermo-dynamically favored direction for the reaction
(a) What is the equilibrium constant for this reaction at 25 °C

(b) If a mixture was prepared containing 1 M glucose-6-phosphate and 1 × 10 3 M glucose-1-phosphate, what would be the thermo-dynamically favored direction for the reaction
Explanation
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


