
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Edition 4ISBN: 978-0138004644
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Edition 4ISBN: 978-0138004644 Exercise 3
Freshly prepared mitochondria were incubated with -hydroxybu-tyrate, oxidized cytochrome c, ADP, P i , and cyanide. -Hydroxybutyrate is oxidized by an NAD + -dependent dehydrogenase. 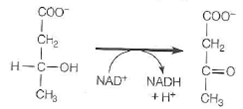
The experimenter measured the rate of oxidation of -hydroxybutyrate and the rate of formation of ATP.
(a) Indicate the probable flow of electrons in this system.
(b) How many moles of ATP would you expect to be formed per mole of -hydroxybutyrate oxidized in this system
(c) Why is -hydroxybutyrate added rather than NADH
(d) What is the function of the cyanide
(e) Write a balanced equation for the overall reaction occurring in this system (electron transport and ATP synthesis).
(f) Calculate the net standard free energy change ( G ° ) in this system, using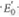 values from Table 15.1 and a G ° value for ATP hydrolysis of -30.5 kJ/mol.
values from Table 15.1 and a G ° value for ATP hydrolysis of -30.5 kJ/mol.

The experimenter measured the rate of oxidation of -hydroxybutyrate and the rate of formation of ATP.
(a) Indicate the probable flow of electrons in this system.
(b) How many moles of ATP would you expect to be formed per mole of -hydroxybutyrate oxidized in this system
(c) Why is -hydroxybutyrate added rather than NADH
(d) What is the function of the cyanide
(e) Write a balanced equation for the overall reaction occurring in this system (electron transport and ATP synthesis).
(f) Calculate the net standard free energy change ( G ° ) in this system, using
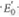 values from Table 15.1 and a G ° value for ATP hydrolysis of -30.5 kJ/mol.
values from Table 15.1 and a G ° value for ATP hydrolysis of -30.5 kJ/mol.Explanation
(a)In oxidation of -Hydroxybutyric acid ...
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


