
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Edition 4ISBN: 978-0138004644
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Edition 4ISBN: 978-0138004644 Exercise 12
GSSG + NADPH + H + 2GSH + NADP +
(a) Calculate G ° for the glutathione reductase reaction in the direction shown, using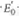 values from Table 15.1.
values from Table 15.1.
(b) Suppose that a cell contained an isoform of glutathione reductase that used NADH instead of NADPH as the reductive coenzyme. Would you expect G ° for this enzyme to be higher, lower, or the same as the corresponding value for the real glutathione reductase Briefly expiam your answer.
(c) Given what you know about the metabolic roles and/or intracellular concentration ratios of NAD + / NADH and NADP + / NADPH, would you expect AG (not G ° ) for this enzyme to be higher, lower, or the same as G for the real enzyme under intracellular conditions Briefly explain your answer.
(a) Calculate G ° for the glutathione reductase reaction in the direction shown, using
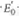 values from Table 15.1.
values from Table 15.1.(b) Suppose that a cell contained an isoform of glutathione reductase that used NADH instead of NADPH as the reductive coenzyme. Would you expect G ° for this enzyme to be higher, lower, or the same as the corresponding value for the real glutathione reductase Briefly expiam your answer.
(c) Given what you know about the metabolic roles and/or intracellular concentration ratios of NAD + / NADH and NADP + / NADPH, would you expect AG (not G ° ) for this enzyme to be higher, lower, or the same as G for the real enzyme under intracellular conditions Briefly explain your answer.
Explanation
(a)
The
value for the glutathione red...
Biochemistry 4th Edition by Christopher Mathews,Kensal van Holde, Dean Appling, Spencer Anthony Cahill
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


