Becker's World of the Cell 9th Edition by Lewis Kleinsmith, Jeff Hardin, Gregory Paul Bertoni
Edition 9ISBN: 9780134295510
Becker's World of the Cell 9th Edition by Lewis Kleinsmith, Jeff Hardin, Gregory Paul Bertoni
Edition 9ISBN: 9780134295510 Exercise 7
Calculating G°' and G'. Like Reaction 5-28, the conversion of 3-phosphoglycerate (3PG)to 2-phosphoglycerate (2PG)is an important cellular reaction because it is one of the steps in the glycolytic pathway:
 (1)
(1)
If the enzyme that catalyzes this reaction is added to a solution of 3PG at 25°C and pH 7.0, the equilibrium ratio between the two species will be 0.165:
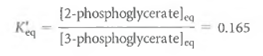 (2)
(2)
Experimental values for the actual steady-state concentrations of these compounds in human red blood cells are 61 M for 3PG and 4.3 M for 2PG.
(a)Calculate G°'. Explain in your own words what this value means.
(b)Calculate G'. Explain in your own words what this value means. Why are G' and G°' different
(c)If conditions in the cell change such that the concentration of 3PG remains fixed at 61 M but the concentration of 2PG begins to rise, how high can the 2PG concentration get before Reaction 5-29 will cease because it is no longer thermodynamically feasible
 (1)
(1)If the enzyme that catalyzes this reaction is added to a solution of 3PG at 25°C and pH 7.0, the equilibrium ratio between the two species will be 0.165:
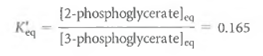 (2)
(2)Experimental values for the actual steady-state concentrations of these compounds in human red blood cells are 61 M for 3PG and 4.3 M for 2PG.
(a)Calculate G°'. Explain in your own words what this value means.
(b)Calculate G'. Explain in your own words what this value means. Why are G' and G°' different
(c)If conditions in the cell change such that the concentration of 3PG remains fixed at 61 M but the concentration of 2PG begins to rise, how high can the 2PG concentration get before Reaction 5-29 will cease because it is no longer thermodynamically feasible
Explanation
(a)
Biochemical standard free energy
...
Becker's World of the Cell 9th Edition by Lewis Kleinsmith, Jeff Hardin, Gregory Paul Bertoni
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


