Becker's World of the Cell 9th Edition by Lewis Kleinsmith, Jeff Hardin, Gregory Paul Bertoni
Edition 9ISBN: 9780134295510
Becker's World of the Cell 9th Edition by Lewis Kleinsmith, Jeff Hardin, Gregory Paul Bertoni
Edition 9ISBN: 9780134295510 Exercise 12
Proof of Additivity. A useful property of thermodynamic parameters such as G' or G°' is that they are additive for sequential reactions. Assume that K' AB , K ' BC , and K' CD are the respective equilibrium constants for reactions 1, 2, and 3 of the following sequence:
 (1)
(1)
(a)Prove that the equilibrium constant K' AD for the overall conversion of A to D is the product of the three component equilibrium constants:
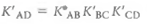 (2)
(2)
(b)Prove that the G°' for the overall conversion of A to D is the sum of the three component G°' values:
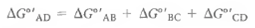 (3)
(3)
(c)Prove that the G'' values are similarly additive.
 (1)
(1)(a)Prove that the equilibrium constant K' AD for the overall conversion of A to D is the product of the three component equilibrium constants:
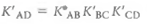 (2)
(2)(b)Prove that the G°' for the overall conversion of A to D is the sum of the three component G°' values:
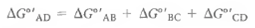 (3)
(3)(c)Prove that the G'' values are similarly additive.
Explanation
(a)
The equilibrium constant for a react...
Becker's World of the Cell 9th Edition by Lewis Kleinsmith, Jeff Hardin, Gregory Paul Bertoni
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


