Becker's World of the Cell 9th Edition by Lewis Kleinsmith, Jeff Hardin, Gregory Paul Bertoni
Edition 9ISBN: 9780134295510
Becker's World of the Cell 9th Edition by Lewis Kleinsmith, Jeff Hardin, Gregory Paul Bertoni
Edition 9ISBN: 9780134295510 Exercise 13
Utilizing Additivity. The additivity of thermodynamic parameters discussed in Problem 5-11 applies not just to sequential reactions in a pathway, but to any reactions or processes. Moreover, it applies to subtraction of reactions. Use this information to answer the following questions:
(a)The phosphorylation of glucose using inorganic phosphate (abbreviated P 1 )is endergonic ( G° = +3.3 kcal/mol), whereas the dephosphorylation (hydrolysis)of ATP is exergonic ( G ° = 7.3 kcal/mol):
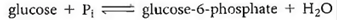

Write a reaction for the phosphorylation of glucose by the transfer of a phosphate group from ATP, and calculate G ° for the reaction.
(b)Phosphocreatine is used by your muscle cells to store energy. The dephosphorylation of phosphocreatine (Reaction 5-40), like that of ATP, is a highly exergonic reaction with G ° = 10.3 kcal/mol:
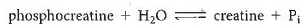
Write a reaction for the transfer of phosphate from phosphocreatineto ADP to generate creatine and ATP, and calculate G ° for the reaction.
(a)The phosphorylation of glucose using inorganic phosphate (abbreviated P 1 )is endergonic ( G° = +3.3 kcal/mol), whereas the dephosphorylation (hydrolysis)of ATP is exergonic ( G ° = 7.3 kcal/mol):
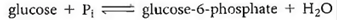

Write a reaction for the phosphorylation of glucose by the transfer of a phosphate group from ATP, and calculate G ° for the reaction.
(b)Phosphocreatine is used by your muscle cells to store energy. The dephosphorylation of phosphocreatine (Reaction 5-40), like that of ATP, is a highly exergonic reaction with G ° = 10.3 kcal/mol:
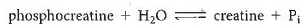
Write a reaction for the transfer of phosphate from phosphocreatineto ADP to generate creatine and ATP, and calculate G ° for the reaction.
Explanation
(a)
A reaction for the complete process ...
Becker's World of the Cell 9th Edition by Lewis Kleinsmith, Jeff Hardin, Gregory Paul Bertoni
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


