Becker's World of the Cell 9th Edition by Lewis Kleinsmith, Jeff Hardin, Gregory Paul Bertoni
Edition 9ISBN: 9780134295510
Becker's World of the Cell 9th Edition by Lewis Kleinsmith, Jeff Hardin, Gregory Paul Bertoni
Edition 9ISBN: 9780134295510 Exercise 5
Glucose Phosphorylation. The direct phosphorylation of glucose by inorganic phosphate is a thermodynamically unfavorable reaction:
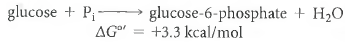 (1)
(1)
In the cell, glucose phosphorylation is accomplished by coupling the reaction to the hydrolysis of ATP, a highly exergonic reaction:
 (2)
(2)
Typical concentrations of these intermediates in yeast cells are as follows:

Assume a temperature of 25°C for all calculations.
(a)What minimum concentration of glucose would have to be maintained in a yeast cell for direct phosphorylation (Reaction 9-21)to be thermodynamically spontaneous Is this physiologically reasonable Explain your reasoning.
(b)What is the overall equation for the coupled (ATP-driven)phosphorylation of glucose What is its G ° value
(c)What minimum concentration of glucose would have to be maintained in a yeast cell for the coupled reaction to be thermodynamically spontaneous Is this physiologically reasonable
(d)By about how many orders of magnitude is the minimum required glucose concentration reduced when the phosphorylation of glucose is coupled to the hydrolysis of ATP
(e)Assuming a yeast cell to have a glucose concentration of 5.0 mM, what is G for the coupled phosphorylation reaction
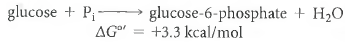 (1)
(1)In the cell, glucose phosphorylation is accomplished by coupling the reaction to the hydrolysis of ATP, a highly exergonic reaction:
 (2)
(2)Typical concentrations of these intermediates in yeast cells are as follows:

Assume a temperature of 25°C for all calculations.
(a)What minimum concentration of glucose would have to be maintained in a yeast cell for direct phosphorylation (Reaction 9-21)to be thermodynamically spontaneous Is this physiologically reasonable Explain your reasoning.
(b)What is the overall equation for the coupled (ATP-driven)phosphorylation of glucose What is its G ° value
(c)What minimum concentration of glucose would have to be maintained in a yeast cell for the coupled reaction to be thermodynamically spontaneous Is this physiologically reasonable
(d)By about how many orders of magnitude is the minimum required glucose concentration reduced when the phosphorylation of glucose is coupled to the hydrolysis of ATP
(e)Assuming a yeast cell to have a glucose concentration of 5.0 mM, what is G for the coupled phosphorylation reaction
Explanation
(a)
For a reaction to be thermodynamical...
Becker's World of the Cell 9th Edition by Lewis Kleinsmith, Jeff Hardin, Gregory Paul Bertoni
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


