
Biochemistry by Raymond Ochs
Edition 0ISBN: 978-1449661373
Biochemistry by Raymond Ochs
Edition 0ISBN: 978-1449661373 Exercise 2
In a solution of pure water, it is possible to calculate the concentration of water itself. What is its value?
Explanation
The water molecule consists of two hydrogen atom and one oxygen atom arranged as follows:
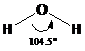 In pure water, the water molecules tend to remain in equilibrium between its stable and protonated state. This can be understood as follows:
In pure water, the water molecules tend to remain in equilibrium between its stable and protonated state. This can be understood as follows:
 Thus, it can be said that one water molecule at present carries proton and exists as hydronium ion or in the protonated (H ₃ O + ) state while others would be in the stable (H ₂ O) state. This proton is transported from one molecule of water to another at a very high rate as compared to diffusion.
Thus, it can be said that one water molecule at present carries proton and exists as hydronium ion or in the protonated (H ₃ O + ) state while others would be in the stable (H ₂ O) state. This proton is transported from one molecule of water to another at a very high rate as compared to diffusion.
Thus, it can be said that above 90 % of water concentration can be found as water (H ₂ O).
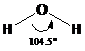 In pure water, the water molecules tend to remain in equilibrium between its stable and protonated state. This can be understood as follows:
In pure water, the water molecules tend to remain in equilibrium between its stable and protonated state. This can be understood as follows: Thus, it can be said that one water molecule at present carries proton and exists as hydronium ion or in the protonated (H ₃ O + ) state while others would be in the stable (H ₂ O) state. This proton is transported from one molecule of water to another at a very high rate as compared to diffusion.
Thus, it can be said that one water molecule at present carries proton and exists as hydronium ion or in the protonated (H ₃ O + ) state while others would be in the stable (H ₂ O) state. This proton is transported from one molecule of water to another at a very high rate as compared to diffusion. Thus, it can be said that above 90 % of water concentration can be found as water (H ₂ O).
Biochemistry by Raymond Ochs
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


