
College Physics 10th Edition by Raymond Serway,Chris Vuille
Edition 10ISBN: 978-1285737041
College Physics 10th Edition by Raymond Serway,Chris Vuille
Edition 10ISBN: 978-1285737041 Exercise 5
Operation of the pulse oximeter (see previous problem). The transmission of light energy as it passes through a solution of light-absorbing molecules is described by the Beer Lambert law 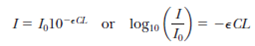
which gives the decrease in intensity I in terms of the distance L the light has traveled through a fluid with a concentration C of the light-absorbing molecule. The quantity is called the extinction coefficient, and its value depends on the frequency of the light. (It has units of m 2 /mol.) Assume the extinction coefficient for 660-nm light passing through a solution of oxygenated hemoglobin is identical to the coefficient for 940-nm light passing through deoxygenated hemoglobin. Also assume 940-nm light has zero absorption ( = 0) in oxygenated hemoglobin and 660-nm light has zero absorption in deoxygenated hemoglobin. If 33% of the energy of the red source and 76% of the infrared energy is transmitted through the blood, what is the fraction of hemoglobin that is oxygenated
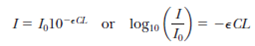
which gives the decrease in intensity I in terms of the distance L the light has traveled through a fluid with a concentration C of the light-absorbing molecule. The quantity is called the extinction coefficient, and its value depends on the frequency of the light. (It has units of m 2 /mol.) Assume the extinction coefficient for 660-nm light passing through a solution of oxygenated hemoglobin is identical to the coefficient for 940-nm light passing through deoxygenated hemoglobin. Also assume 940-nm light has zero absorption ( = 0) in oxygenated hemoglobin and 660-nm light has zero absorption in deoxygenated hemoglobin. If 33% of the energy of the red source and 76% of the infrared energy is transmitted through the blood, what is the fraction of hemoglobin that is oxygenated
Explanation
Use the formula of Beer-Lambert Law to s...
College Physics 10th Edition by Raymond Serway,Chris Vuille
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


