
College Physics 10th Edition by Raymond Serway,Chris Vuille
Edition 10ISBN: 978-1285737041
College Physics 10th Edition by Raymond Serway,Chris Vuille
Edition 10ISBN: 978-1285737041 Exercise 19
A general expression for the energy levels of oneelectron atoms and ions is 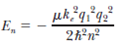
Here µ is the reduced mass of the atom, given by µ = m 1 m 2 /( m 1 + m 2 ), where m 1 is the mass of the electron and m 2 is the mass of the nucleus; k e is the Coulomb constant; and q 1 and q 2 are the charges of the electron and the nucleus, respectively. The wavelength for the n = 3 to n = 2 transition of the hydrogen atom is 656.3 nm (visible red light). What are the wavelengths for this same transition in (a) positronium, which consists of an electron and a positron, and (b) singly ionized helium Note: A positron is a positively charged electron.
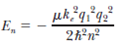
Here µ is the reduced mass of the atom, given by µ = m 1 m 2 /( m 1 + m 2 ), where m 1 is the mass of the electron and m 2 is the mass of the nucleus; k e is the Coulomb constant; and q 1 and q 2 are the charges of the electron and the nucleus, respectively. The wavelength for the n = 3 to n = 2 transition of the hydrogen atom is 656.3 nm (visible red light). What are the wavelengths for this same transition in (a) positronium, which consists of an electron and a positron, and (b) singly ionized helium Note: A positron is a positively charged electron.
Explanation
(a)
The energy of the electron that was ...
College Physics 10th Edition by Raymond Serway,Chris Vuille
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


