
Optical Fiber Communications 4th Edition by Gerd Keiser
Edition 4ISBN: 978-0073380711
Optical Fiber Communications 4th Edition by Gerd Keiser
Edition 4ISBN: 978-0073380711 Exercise 2
What are the energies in electron volts (eV) of light at wavelengths 850, 1310, 1490, and 1550 nm ( b ) Consider a 1-ns pulse with a 100-nW amplitude at each of these wavelengths. How many photons are in such a pulse at each wavelength
Explanation
(a)
Write the expression of energy in terms of electron volts.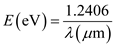 …… (1)
…… (1)
Determine the value of energy for the wave length 850 nm.
Substitute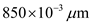 for
for 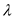 in equation (1).
in equation (1).  Therefore, the value of energy for the wave length 850 nm is
Therefore, the value of energy for the wave length 850 nm is 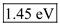 .
.
Determine the value of energy for the wave length 1310 nm.
Substitute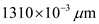 for
for 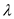 in equation (1).
in equation (1).  Therefore, the value of energy for the wave length 1310 nm is
Therefore, the value of energy for the wave length 1310 nm is 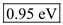 .
.
Determine the value of energy for the wave length 1490 nm.
Substitute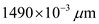 for
for 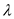 in equation (1).
in equation (1).  Therefore, the value of energy for the wave length 1490 nm is
Therefore, the value of energy for the wave length 1490 nm is 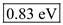 .
.
Determine the value of energy for the wave length 1550 nm.
Substitute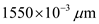 for
for 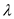 in equation (1).
in equation (1).  Therefore, the value of energy for the wave length 1550 nm is
Therefore, the value of energy for the wave length 1550 nm is 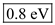 .
.
(b)
Write the expression to calculate number of photons.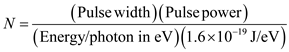 …… (2)
…… (2)
Determine the number of photons for 850 nm.
Substitute 1ns for pulse width, 100-nW for pulse power and1.45 eV for Energy in equation (2). Therefore, the number of photons for 850 nm is
Therefore, the number of photons for 850 nm is 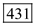 .
.
Determine the number of photons for 1310 nm.
Substitute 1ns for pulse width, 100-nW for pulse power and 0.95eV for Energy in equation (2). Therefore, the number of photons for 1310 nm is
Therefore, the number of photons for 1310 nm is 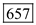 .
.
Determine the number of photons for 1490 nm.
Substitute 1ns for pulse width, 100-nW for pulse power and 0.8 eV for Energy in equation (2). Therefore, the number of photons for 1490 nm is
Therefore, the number of photons for 1490 nm is 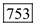 .
.
Determine the number of photons for 1550 nm.
Substitute 1ns for pulse width, 100-nW for pulse power and 0.83eV for Energy in equation (2). Therefore, the number of photons for 1550 nm is
Therefore, the number of photons for 1550 nm is 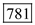 .
.
Write the expression of energy in terms of electron volts.
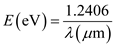 …… (1)
…… (1)Determine the value of energy for the wave length 850 nm.
Substitute
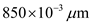 for
for 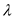 in equation (1).
in equation (1).  Therefore, the value of energy for the wave length 850 nm is
Therefore, the value of energy for the wave length 850 nm is 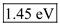 .
.Determine the value of energy for the wave length 1310 nm.
Substitute
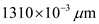 for
for 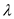 in equation (1).
in equation (1).  Therefore, the value of energy for the wave length 1310 nm is
Therefore, the value of energy for the wave length 1310 nm is 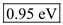 .
.Determine the value of energy for the wave length 1490 nm.
Substitute
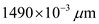 for
for 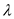 in equation (1).
in equation (1).  Therefore, the value of energy for the wave length 1490 nm is
Therefore, the value of energy for the wave length 1490 nm is 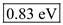 .
.Determine the value of energy for the wave length 1550 nm.
Substitute
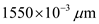 for
for 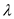 in equation (1).
in equation (1).  Therefore, the value of energy for the wave length 1550 nm is
Therefore, the value of energy for the wave length 1550 nm is 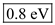 .
.(b)
Write the expression to calculate number of photons.
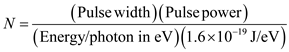 …… (2)
…… (2)Determine the number of photons for 850 nm.
Substitute 1ns for pulse width, 100-nW for pulse power and1.45 eV for Energy in equation (2).
 Therefore, the number of photons for 850 nm is
Therefore, the number of photons for 850 nm is 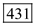 .
.Determine the number of photons for 1310 nm.
Substitute 1ns for pulse width, 100-nW for pulse power and 0.95eV for Energy in equation (2).
 Therefore, the number of photons for 1310 nm is
Therefore, the number of photons for 1310 nm is 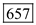 .
.Determine the number of photons for 1490 nm.
Substitute 1ns for pulse width, 100-nW for pulse power and 0.8 eV for Energy in equation (2).
 Therefore, the number of photons for 1490 nm is
Therefore, the number of photons for 1490 nm is 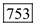 .
.Determine the number of photons for 1550 nm.
Substitute 1ns for pulse width, 100-nW for pulse power and 0.83eV for Energy in equation (2).
 Therefore, the number of photons for 1550 nm is
Therefore, the number of photons for 1550 nm is 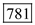 .
.Optical Fiber Communications 4th Edition by Gerd Keiser
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


