
Environmental Science 15th Edition by Scott Spoolman,Tyler Miller
Edition 15ISBN: 978-1305090446
Environmental Science 15th Edition by Scott Spoolman,Tyler Miller
Edition 15ISBN: 978-1305090446 Exercise 1
OCEAN ACIDIFICATION: THE OTHER CO2 PROBLEM
By burning an increasingly large amount of carbon-containing fossil fuels, especially since 1950, we have added carbon dioxide (CO2 ) to the atmosphere faster than it can be removed by the carbon cycle (see Figure 3.14, p. 53). Globally, CO2 emissions have risen by about 45% between 1992 and 2013. Extensive research indicates that if we continue to increase CO2 levels in the atmosphere, we will play a role in disrupting the earth's climate during this century.
Another problem related to CO2 emissions is ocean acidification, a change in ocean chemistry. The oceans have absorbed about one-fourth of the excess CO2 that human activities have added to the atmosphere. When this absorbed CO2 combines with ocean water, it forms carbonic acid (H2 CO3 ), a weak acid also found in carbonated drinks. As a result, the level of hydrogen ions (H + ) in the water rises, making the water less basic, while the level of carbonate ions (CO3 2? ) in the water drops because these ions react with hydrogen ions (H + ) to form bicarbonate ions (HCO3 ? ).
The problem is that many aquatic species, including phytoplankton, corals, sea snails, crabs, and oysters, use carbonate ions to produce calcium carbonate (CaCO3 ), the main component of their shells and bones. As a result, in less basic waters, shell-building species and coral reefs grow more slowly (Figure 9.C), and when the hydrogen ion concentration of the surrounding seawater gets high enough, their calcium carbonate begins to dissolve. These effects will occur first in colder ocean waters, because they have a greater capacity for absorption of CO2 from the atmosphere than warmer waters have.
Since we began burning fossil fuels in large quantities during the Industrial Revolution of the 18th and 19th centuries, there has been a 30% rise in the average acidity (actually a 30% decrease in average basicity) of surface ocean water, according to a 2010 UNEP summary of research on this problem. A 2013 report prepared by 540 scientists from 37 countries projected that, by 2100, we could see a 170% drop in the average basicity of surface ocean water because of increasing acidity levels. The report also warned that this would reduce the ability of the oceans to help in regulating the rate of climate change by absorbing CO2 , and that it could jeopardize the stability of marine ecosystems and lead to a loss of some $130 billion a year for the fishing industry.
According to most marine scientists, the only way to slow these changes is through a quick and sharp reduction in the use of fossil fuels around the world, which would lessen the massive inputs of CO2 into the air and from there into the ocean. We can also slow the rise of acidity levels in ocean waters by protecting and restoring mangrove forests, sea grasses, and coastal wetlands, because these aquatic systems take up and store some of the atmospheric CO2 that is at the heart of this problem.
Critical Thinking
How might widespread losses of some forms of marine aquatic life due to ocean acidification affect life on land? How might it affect your life? (Hint: Think food webs.)
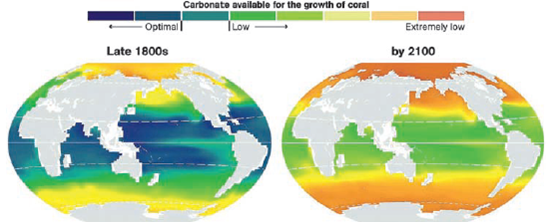
FIGURE 9.C Calcium carbonate levels in ocean waters, calculated from historical data (left), and projected for 2100 (right). Colors shifting from blue to red indicate waters becoming less basic.
Sources: Andrew G. Dickson, Scripps Institution of Oceanography, U.C. San Diego, and Sarah Cooley, Woods Hole Oceanographic Institution. Used by permission from National Geographic.
National Geographic Maps/National Geographic Creative
By burning an increasingly large amount of carbon-containing fossil fuels, especially since 1950, we have added carbon dioxide (CO2 ) to the atmosphere faster than it can be removed by the carbon cycle (see Figure 3.14, p. 53). Globally, CO2 emissions have risen by about 45% between 1992 and 2013. Extensive research indicates that if we continue to increase CO2 levels in the atmosphere, we will play a role in disrupting the earth's climate during this century.
Another problem related to CO2 emissions is ocean acidification, a change in ocean chemistry. The oceans have absorbed about one-fourth of the excess CO2 that human activities have added to the atmosphere. When this absorbed CO2 combines with ocean water, it forms carbonic acid (H2 CO3 ), a weak acid also found in carbonated drinks. As a result, the level of hydrogen ions (H + ) in the water rises, making the water less basic, while the level of carbonate ions (CO3 2? ) in the water drops because these ions react with hydrogen ions (H + ) to form bicarbonate ions (HCO3 ? ).
The problem is that many aquatic species, including phytoplankton, corals, sea snails, crabs, and oysters, use carbonate ions to produce calcium carbonate (CaCO3 ), the main component of their shells and bones. As a result, in less basic waters, shell-building species and coral reefs grow more slowly (Figure 9.C), and when the hydrogen ion concentration of the surrounding seawater gets high enough, their calcium carbonate begins to dissolve. These effects will occur first in colder ocean waters, because they have a greater capacity for absorption of CO2 from the atmosphere than warmer waters have.
Since we began burning fossil fuels in large quantities during the Industrial Revolution of the 18th and 19th centuries, there has been a 30% rise in the average acidity (actually a 30% decrease in average basicity) of surface ocean water, according to a 2010 UNEP summary of research on this problem. A 2013 report prepared by 540 scientists from 37 countries projected that, by 2100, we could see a 170% drop in the average basicity of surface ocean water because of increasing acidity levels. The report also warned that this would reduce the ability of the oceans to help in regulating the rate of climate change by absorbing CO2 , and that it could jeopardize the stability of marine ecosystems and lead to a loss of some $130 billion a year for the fishing industry.
According to most marine scientists, the only way to slow these changes is through a quick and sharp reduction in the use of fossil fuels around the world, which would lessen the massive inputs of CO2 into the air and from there into the ocean. We can also slow the rise of acidity levels in ocean waters by protecting and restoring mangrove forests, sea grasses, and coastal wetlands, because these aquatic systems take up and store some of the atmospheric CO2 that is at the heart of this problem.
Critical Thinking
How might widespread losses of some forms of marine aquatic life due to ocean acidification affect life on land? How might it affect your life? (Hint: Think food webs.)

FIGURE 9.C Calcium carbonate levels in ocean waters, calculated from historical data (left), and projected for 2100 (right). Colors shifting from blue to red indicate waters becoming less basic.
Sources: Andrew G. Dickson, Scripps Institution of Oceanography, U.C. San Diego, and Sarah Cooley, Woods Hole Oceanographic Institution. Used by permission from National Geographic.
National Geographic Maps/National Geographic Creative
Explanation
A lot of land animals are dependent on m...
Environmental Science 15th Edition by Scott Spoolman,Tyler Miller
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


