
Campbell Biology 11th Edition by Lisa Urry,Michael Cain,Steven Wasserman,Peter Minorsky,Jane Reece
Edition 11ISBN: 978-0134093413
Campbell Biology 11th Edition by Lisa Urry,Michael Cain,Steven Wasserman,Peter Minorsky,Jane Reece
Edition 11ISBN: 978-0134093413 Exercise 12
Working with Moles and Molar Ratios
Could the First Biological Molecules Have Formed Near Volcanoes on Early Earth In 2007, Jeffrey Bada, a former graduate student of Stanley Miller, discovered some vials of samples that had never been analyzed from an experiment performed by Miller in 1958. In that experiment, Miller used hydrogen sulfide gas (H₂ S) as one of the gases in the reactant mixture. Since H₂ S is released by volcanoes, the H₂ S experiment was designed to mimic conditions near volcanoes on early Earth. In 2011, Bada and colleagues published the results of their analysis of these "lost" samples. In this exercise, you will make calculations using the molar ratios of reactants and products from the H₂ S experiment.
How the Experiment Was Done According to his laboratory notebook, Miller used the same apparatus as in his original experiment (see Figure 4.2), but the mixture of gaseous reactants included methane (CH₄), carbon dioxide (CO₂ ), hydrogen sulfide (H₂ S), and ammonia (NH 3 ). After three days of simulated volcanic activity, he collected samples of the liquid, partially purified the chemicals, and sealed the samples in sterile vials. In 2011, Bada's research team used modern analytical methods to analyze the products in the vials for the presence of amino acids, the building blocks of proteins.
Data from the Experiment The table below shows 4 of the 23 amino acids detected in the 2011 analysis of the samples from Miller's 1958 H₂ S experiment.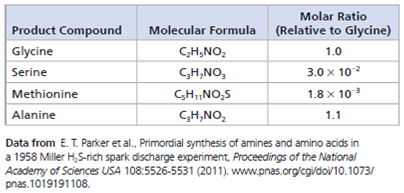

(a) Which amino acid is present in higher amounts than glycine
(b) How many more molecules of that amino acid are present than the number of molecules in 1.0 mole of glycine
Could the First Biological Molecules Have Formed Near Volcanoes on Early Earth In 2007, Jeffrey Bada, a former graduate student of Stanley Miller, discovered some vials of samples that had never been analyzed from an experiment performed by Miller in 1958. In that experiment, Miller used hydrogen sulfide gas (H₂ S) as one of the gases in the reactant mixture. Since H₂ S is released by volcanoes, the H₂ S experiment was designed to mimic conditions near volcanoes on early Earth. In 2011, Bada and colleagues published the results of their analysis of these "lost" samples. In this exercise, you will make calculations using the molar ratios of reactants and products from the H₂ S experiment.
How the Experiment Was Done According to his laboratory notebook, Miller used the same apparatus as in his original experiment (see Figure 4.2), but the mixture of gaseous reactants included methane (CH₄), carbon dioxide (CO₂ ), hydrogen sulfide (H₂ S), and ammonia (NH 3 ). After three days of simulated volcanic activity, he collected samples of the liquid, partially purified the chemicals, and sealed the samples in sterile vials. In 2011, Bada's research team used modern analytical methods to analyze the products in the vials for the presence of amino acids, the building blocks of proteins.
Data from the Experiment The table below shows 4 of the 23 amino acids detected in the 2011 analysis of the samples from Miller's 1958 H₂ S experiment.


(a) Which amino acid is present in higher amounts than glycine
(b) How many more molecules of that amino acid are present than the number of molecules in 1.0 mole of glycine
Explanation
In the given data, the amount of amino a...
Campbell Biology 11th Edition by Lisa Urry,Michael Cain,Steven Wasserman,Peter Minorsky,Jane Reece
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


