
Molecular Biology Of The Cell 6th Edition by Bruce Alberts, Alexander Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Edition 6ISBN: 978-0815345244
Molecular Biology Of The Cell 6th Edition by Bruce Alberts, Alexander Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Edition 6ISBN: 978-0815345244 Exercise 7
Titin, which has a molecular weight of about 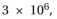 is the largest polypeptide yet described. Titin molecules extend from muscle thick filaments to the Z disc; they are thought to act as springs to keep the thick filaments centered in the sarcomere. Titin is composed of a large number of repeated immunoglobulin (Ig) sequences of 89 amino acids, each of which is folded into a domain about 4 nm in length (Figure Q3-2A). You suspect that the springlike behavior of titin is caused by the sequential unfolding (and refolding) of indi- vidual Ig domains. You test this hypothesis using the atomic force microscope, which allows you to pick up one end of a protein molecule and pull with an accurately measured force. For a fragment of titin containing seven repeats of the Ig domain, this experiment gives the sawtooth force-ver- sus-extension curve shown in Figure Q3-2 b. If the experi- ment is repeated in a solution of 8 M urea (a protein dena- turant), the peaks disappear and the measured extension becomes much longer for a given force. If the experiment is repeated after the protein has been cross-linked by treat- ment with glutaraldehyde, once again the peaks disappear but the extension becomes much smaller for a given force.
is the largest polypeptide yet described. Titin molecules extend from muscle thick filaments to the Z disc; they are thought to act as springs to keep the thick filaments centered in the sarcomere. Titin is composed of a large number of repeated immunoglobulin (Ig) sequences of 89 amino acids, each of which is folded into a domain about 4 nm in length (Figure Q3-2A). You suspect that the springlike behavior of titin is caused by the sequential unfolding (and refolding) of indi- vidual Ig domains. You test this hypothesis using the atomic force microscope, which allows you to pick up one end of a protein molecule and pull with an accurately measured force. For a fragment of titin containing seven repeats of the Ig domain, this experiment gives the sawtooth force-ver- sus-extension curve shown in Figure Q3-2 b. If the experi- ment is repeated in a solution of 8 M urea (a protein dena- turant), the peaks disappear and the measured extension becomes much longer for a given force. If the experiment is repeated after the protein has been cross-linked by treat- ment with glutaraldehyde, once again the peaks disappear but the extension becomes much smaller for a given force. 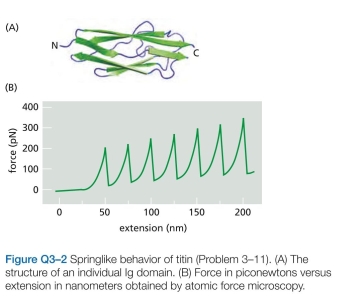 a. Are the data consistent with your hypothesis that titin's springlike behavior is due to the sequential unfold- ing of individual Ig domains? Explain your reasoning. b. Is the extension for each putative domain-un- folding event the magnitude you would expect? (In an extended polypeptide chain, amino acids are spaced at intervals of 0.34 nm.)
a. Are the data consistent with your hypothesis that titin's springlike behavior is due to the sequential unfold- ing of individual Ig domains? Explain your reasoning. b. Is the extension for each putative domain-un- folding event the magnitude you would expect? (In an extended polypeptide chain, amino acids are spaced at intervals of 0.34 nm.)
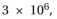 is the largest polypeptide yet described. Titin molecules extend from muscle thick filaments to the Z disc; they are thought to act as springs to keep the thick filaments centered in the sarcomere. Titin is composed of a large number of repeated immunoglobulin (Ig) sequences of 89 amino acids, each of which is folded into a domain about 4 nm in length (Figure Q3-2A). You suspect that the springlike behavior of titin is caused by the sequential unfolding (and refolding) of indi- vidual Ig domains. You test this hypothesis using the atomic force microscope, which allows you to pick up one end of a protein molecule and pull with an accurately measured force. For a fragment of titin containing seven repeats of the Ig domain, this experiment gives the sawtooth force-ver- sus-extension curve shown in Figure Q3-2 b. If the experi- ment is repeated in a solution of 8 M urea (a protein dena- turant), the peaks disappear and the measured extension becomes much longer for a given force. If the experiment is repeated after the protein has been cross-linked by treat- ment with glutaraldehyde, once again the peaks disappear but the extension becomes much smaller for a given force.
is the largest polypeptide yet described. Titin molecules extend from muscle thick filaments to the Z disc; they are thought to act as springs to keep the thick filaments centered in the sarcomere. Titin is composed of a large number of repeated immunoglobulin (Ig) sequences of 89 amino acids, each of which is folded into a domain about 4 nm in length (Figure Q3-2A). You suspect that the springlike behavior of titin is caused by the sequential unfolding (and refolding) of indi- vidual Ig domains. You test this hypothesis using the atomic force microscope, which allows you to pick up one end of a protein molecule and pull with an accurately measured force. For a fragment of titin containing seven repeats of the Ig domain, this experiment gives the sawtooth force-ver- sus-extension curve shown in Figure Q3-2 b. If the experi- ment is repeated in a solution of 8 M urea (a protein dena- turant), the peaks disappear and the measured extension becomes much longer for a given force. If the experiment is repeated after the protein has been cross-linked by treat- ment with glutaraldehyde, once again the peaks disappear but the extension becomes much smaller for a given force.  a. Are the data consistent with your hypothesis that titin's springlike behavior is due to the sequential unfold- ing of individual Ig domains? Explain your reasoning. b. Is the extension for each putative domain-un- folding event the magnitude you would expect? (In an extended polypeptide chain, amino acids are spaced at intervals of 0.34 nm.)
a. Are the data consistent with your hypothesis that titin's springlike behavior is due to the sequential unfold- ing of individual Ig domains? Explain your reasoning. b. Is the extension for each putative domain-un- folding event the magnitude you would expect? (In an extended polypeptide chain, amino acids are spaced at intervals of 0.34 nm.)Explanation
Whereas the normal Src is confined to 4-...
Molecular Biology Of The Cell 6th Edition by Bruce Alberts, Alexander Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


