
Molecular Biology Of The Cell 6th Edition by Bruce Alberts, Alexander Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Edition 6ISBN: 978-0815345244
Molecular Biology Of The Cell 6th Edition by Bruce Alberts, Alexander Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Edition 6ISBN: 978-0815345244 Exercise 12
1 How much energy is available in visible light? How much energy does sunlight deliver to Earth? How efficient are plants at converting light energy into chemical energy? The answers to these questions provide an important backdrop to the subject of photosynthesis. Each quantum or photon of light has energy 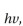 where
where 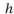 is Planck's constant (6.6 ×
is Planck's constant (6.6 × 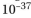 kJ sec/photon) and
kJ sec/photon) and 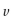 is the frequency in
is the frequency in 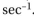 The frequency of light is equal to
The frequency of light is equal to 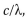 where
where 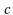 is the speed of light
is the speed of light 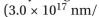 sec) and
sec) and 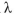 s the wavelength in nm. Thus, the energy (E) of a photon is
s the wavelength in nm. Thus, the energy (E) of a photon is 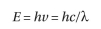
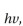 where
where 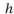 is Planck's constant (6.6 ×
is Planck's constant (6.6 × 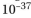 kJ sec/photon) and
kJ sec/photon) and 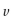 is the frequency in
is the frequency in 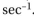 The frequency of light is equal to
The frequency of light is equal to 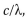 where
where 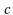 is the speed of light
is the speed of light 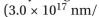 sec) and
sec) and 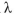 s the wavelength in nm. Thus, the energy (E) of a photon is
s the wavelength in nm. Thus, the energy (E) of a photon is 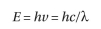
Explanation
Chloroplasts are organelles found in gre...
Molecular Biology Of The Cell 6th Edition by Bruce Alberts, Alexander Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


