
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
Edition 1ISBN: 9780521840996
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
Edition 1ISBN: 9780521840996 Exercise 28
The decomposition of calcium carbonate is shown below along with the standard enthalpy and entropy values.
a. Calculate the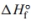 for the reaction.
for the reaction.
b. Calculate the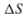 for the reaction.
for the reaction.
c. What is the standard Gibb's free-energy change expression for the reac- tion?
d. Is the reaction spontaneous at 25◦C? Is the reaction spontaneous at 1000◦C? Explain your answers.
e. Calculate the equilibrium constant at 25◦C and 1000◦C.
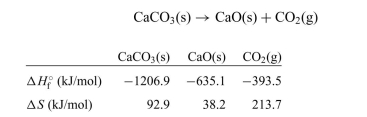
a. Calculate the
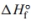 for the reaction.
for the reaction. b. Calculate the
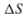 for the reaction.
for the reaction. c. What is the standard Gibb's free-energy change expression for the reac- tion?
d. Is the reaction spontaneous at 25◦C? Is the reaction spontaneous at 1000◦C? Explain your answers.
e. Calculate the equilibrium constant at 25◦C and 1000◦C.

Explanation
a) The ?H o f for the reaction is -2235....
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


