
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
Edition 1ISBN: 9780521840996
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
Edition 1ISBN: 9780521840996 Exercise 30
The reaction in which urea is formed from NH3 and CO2 is shown below. The standard free-energy change 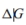 at 25◦Cis−13.6 kJ/mol.
at 25◦Cis−13.6 kJ/mol. 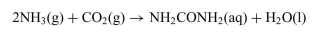
a. Write an expression for the equilibrium constant, K, in terms of the molar concentrations of the reactants and products.
b. Write an expression for the equilibrium constant, K, as a function of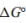 and temperature.
and temperature.
c. Determine the value of the equilibrium constant, K, for this reaction at 25◦C.
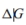 at 25◦Cis−13.6 kJ/mol.
at 25◦Cis−13.6 kJ/mol. 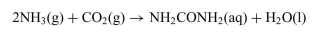
a. Write an expression for the equilibrium constant, K, in terms of the molar concentrations of the reactants and products.
b. Write an expression for the equilibrium constant, K, as a function of
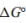 and temperature.
and temperature. c. Determine the value of the equilibrium constant, K, for this reaction at 25◦C.
Explanation
a) Expression for the equilibrium consta...
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


