
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
Edition 1ISBN: 9780521840996
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
Edition 1ISBN: 9780521840996 Exercise 4
For the dissociation reaction of a weak acid shown below, begin with defining the Ka and show all the steps for the derivation of the Henderson-Hasselbalch equation. 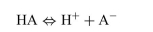 The Henderson-Hasselbalch equation for the blood bicarbonate system is shown as follows:
The Henderson-Hasselbalch equation for the blood bicarbonate system is shown as follows: 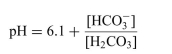
a. Calculate the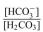 ratio for a blood pH of 5.8.
ratio for a blood pH of 5.8.
b. Is this patient experiencing acidosis or alkalosis? Why?
c. What can the body due to restore the blood pH to normal?
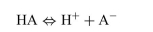 The Henderson-Hasselbalch equation for the blood bicarbonate system is shown as follows:
The Henderson-Hasselbalch equation for the blood bicarbonate system is shown as follows: 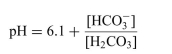
a. Calculate the
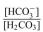 ratio for a blood pH of 5.8.
ratio for a blood pH of 5.8. b. Is this patient experiencing acidosis or alkalosis? Why?
c. What can the body due to restore the blood pH to normal?
Explanation
a. The for a blood at pH = 5.8 is calcu...
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


