
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
Edition 1ISBN: 9780521840996
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
Edition 1ISBN: 9780521840996 Exercise 4
Glutamic acid (1 of the 20 amino acids) has a side-chain carboxyl group 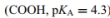 as shown in Figures 4.7 and 4.8.
as shown in Figures 4.7 and 4.8.
a. Write the chemical equation for the dissociation of the side-chain COOH. Label the weak acid and the conjugate base.
b. The Henderson-Hasselbalch equation can be used to determine the ionization status of a weak acid: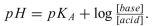 Use the Henderson-Hasselbalch equation to determine whether the glutamic acid side-chain carboxyl group is protonated or deprotonated at physiolo- gical pH.
Use the Henderson-Hasselbalch equation to determine whether the glutamic acid side-chain carboxyl group is protonated or deprotonated at physiolo- gical pH.
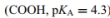 as shown in Figures 4.7 and 4.8.
as shown in Figures 4.7 and 4.8. a. Write the chemical equation for the dissociation of the side-chain COOH. Label the weak acid and the conjugate base.
b. The Henderson-Hasselbalch equation can be used to determine the ionization status of a weak acid:
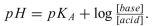 Use the Henderson-Hasselbalch equation to determine whether the glutamic acid side-chain carboxyl group is protonated or deprotonated at physiolo- gical pH.
Use the Henderson-Hasselbalch equation to determine whether the glutamic acid side-chain carboxyl group is protonated or deprotonated at physiolo- gical pH.Explanation
a. The chemical equation for the dissoci...
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


