
Bioprocess Engineering 2nd Edition by Fikret Kargi,Michael Shuler
Edition 2ISBN: 9780130819086
Bioprocess Engineering 2nd Edition by Fikret Kargi,Michael Shuler
Edition 2ISBN: 9780130819086 Exercise 1
An enzyme ATPase has a molecular weight of 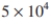 daltons,
daltons, 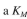 value of 10-4 M, and a K₂
value of 10-4 M, and a K₂
value of K₂ = 104 molecules ATP/min molecule enzyme at 37∞C. The reaction catalyzed is the
following: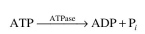 which can also be represented as
which can also be represented as 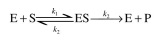 where S is ATP. The enzyme at this temperature is unstable. The enzyme inactivation kinetics
where S is ATP. The enzyme at this temperature is unstable. The enzyme inactivation kinetics
are first order: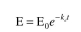 where
where 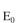 is the initial enzyme concentration and
is the initial enzyme concentration and 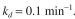 In an experiment with a par-
In an experiment with a par-
tially pure enzyme preparation,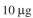 of total crude protein (containing enzyme) is added to a
of total crude protein (containing enzyme) is added to a
1 ml reaction mixture containing 0.02 M ATP and incubated at 37∞C. After 12 hours the reac-
tion ends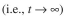 and the inorganic phosphate
and the inorganic phosphate 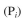 concentration is found to be 0.002 M,
concentration is found to be 0.002 M,
which was initially zero. What fraction of the crude protein preparation was the enzyme?
Hint: Since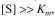 the reaction rate can be represented by
the reaction rate can be represented by 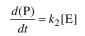
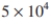 daltons,
daltons, 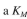 value of 10-4 M, and a K₂
value of 10-4 M, and a K₂value of K₂ = 104 molecules ATP/min molecule enzyme at 37∞C. The reaction catalyzed is the
following:
 which can also be represented as
which can also be represented as 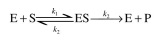 where S is ATP. The enzyme at this temperature is unstable. The enzyme inactivation kinetics
where S is ATP. The enzyme at this temperature is unstable. The enzyme inactivation kineticsare first order:
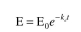 where
where 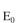 is the initial enzyme concentration and
is the initial enzyme concentration and 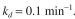 In an experiment with a par-
In an experiment with a par-tially pure enzyme preparation,
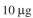 of total crude protein (containing enzyme) is added to a
of total crude protein (containing enzyme) is added to a1 ml reaction mixture containing 0.02 M ATP and incubated at 37∞C. After 12 hours the reac-
tion ends
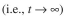 and the inorganic phosphate
and the inorganic phosphate 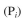 concentration is found to be 0.002 M,
concentration is found to be 0.002 M,which was initially zero. What fraction of the crude protein preparation was the enzyme?
Hint: Since
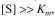 the reaction rate can be represented by
the reaction rate can be represented by 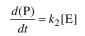
Explanation
The catalyzed reaction is as follows: T...
Bioprocess Engineering 2nd Edition by Fikret Kargi,Michael Shuler
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


