
Bioprocess Engineering 2nd Edition by Fikret Kargi,Michael Shuler
Edition 2ISBN: 9780130819086
Bioprocess Engineering 2nd Edition by Fikret Kargi,Michael Shuler
Edition 2ISBN: 9780130819086 Exercise 14
Two enzymes are both immobilized on the same flat, nonporous surface. For enzyme A the sub-
strate is S₁. For enzyme B the substrate is S₂. The product of the first reaction is S₂. That is: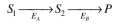
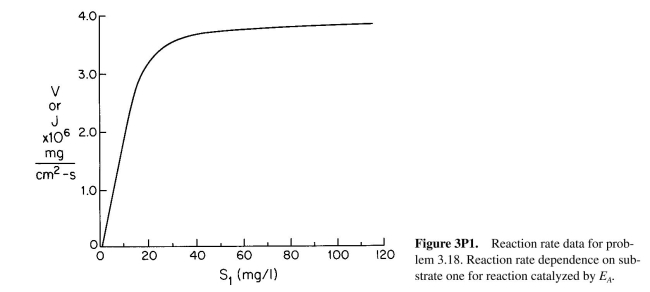
a. Figure 3.P1 depicts the rate of the first reaction on the surface as a function of local con-
centrations of S₁. If the bulk concentration of S₁ is 100 mg/l and the mass transfer coeffi-
cient is
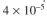 cm/s, what is the rate of consumption of S₁ for a
cm/s, what is the rate of consumption of S₁ for a
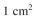 surface? What is
surface? What is
the surface concentration of S₁?
b. The rate of the second reaction is:
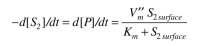
where
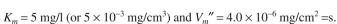 The bulk concentra-
The bulk concentra-
tion of
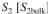 is maintained as 5 mg/l and the mass transfer coefficient is the same for
is maintained as 5 mg/l and the mass transfer coefficient is the same for
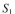
and S₂. Calculate
 and the rate of formation of P (assuming all stoichiometric coeffi-
and the rate of formation of P (assuming all stoichiometric coeffi-
cients are one).
strate is S₁. For enzyme B the substrate is S₂. The product of the first reaction is S₂. That is:
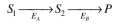
a. Figure 3.P1 depicts the rate of the first reaction on the surface as a function of local con-
centrations of S₁. If the bulk concentration of S₁ is 100 mg/l and the mass transfer coeffi-
cient is
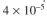 cm/s, what is the rate of consumption of S₁ for a
cm/s, what is the rate of consumption of S₁ for a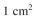 surface? What is
surface? What isthe surface concentration of S₁?
b. The rate of the second reaction is:
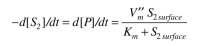

where
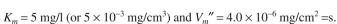 The bulk concentra-
The bulk concentra-tion of
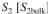 is maintained as 5 mg/l and the mass transfer coefficient is the same for
is maintained as 5 mg/l and the mass transfer coefficient is the same for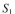
and S₂. Calculate
 and the rate of formation of P (assuming all stoichiometric coeffi-
and the rate of formation of P (assuming all stoichiometric coeffi-cients are one).
Explanation
The given case describes the condition, ...
Bioprocess Engineering 2nd Edition by Fikret Kargi,Michael Shuler
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


